(A) Francium (Z = 87) is an extremely rare radioactive element formed when actinium (Z = 89)...
Question:
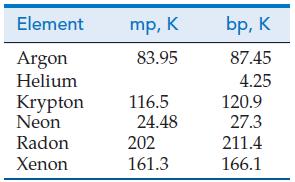
(A) Francium (Z = 87) is an extremely rare radioactive element formed when actinium (Z = 89) undergoes alpha-particle emission. Francium occurs in natural uranium minerals, but estimates are that little more than 15 g of francium exists in the top 1 km of Earth’s crust. Few of francium’s properties have been measured, but some can be inferred from its position in the periodic table. Estimate the melting point, density, and atomic (metallic) radius of francium.
(B) Discuss the likelihood that element 168, should it ever be synthesized in sufficient quantity, would be a “noble liquid” at 298 K and 1 bar. Could element 168 be a “noble solid” at 298 K and 1 bar? Use spdf notation to show the electron configuration you would expect for element 168.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





