A mixture of 1.00 mol NaHCO 3 (s) and 1.00 mol Na 2 CO 3 (s) is
Question:
A mixture of 1.00 mol NaHCO3(s) and 1.00 mol Na2CO3(s) is introduced into a 2.50 L flask in which the partial pressure of CO2 is 2.10 atm and that of H2O(g) is 715 mmHg. When equilibrium is established at 100 °C, will the partial pressures of CO2(g) and H2O(g) be greater or less than their initial partial pressures? Explain.
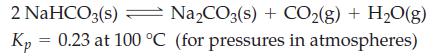
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





