A Ni anode and an Fe cathode are placed in a solution with [Ni 2+ ] =
Question:
A Ni anode and an Fe cathode are placed in a solution with [Ni2+] = 1.0 M and then connected to a battery. The Fe cathode has the shape shown. How long must electrolysis be continued with a current of 1.50 A to build a 0.050-mm-thick deposit of nickel on the iron? (Density of nickel = 8.90 g/cm3.)
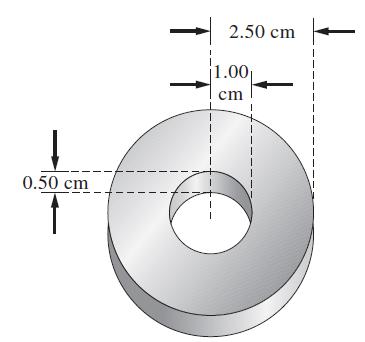
Transcribed Image Text:
0.50 cm 2.50 cm 1.00₁ cm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To determine how long electrolysis must be continued we will need to calculate the mass of nickel th...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A group of investors is considering buying the Wheelwright Corporation, but does not want to contribute to the companys financial support after the purchase. Wheelwrights management has offered the...
-
On January 1, the company purchased a machine for $80,000. The machine had an estimated useful life of eight years and an estimated salvage value of $8,000. After three full years of using the...
-
The first measurement of sea depth was made in 1840 in the central South Atlantic, where a plummet was lowered 2425 fathoms. What is this depth in meters? Note that 1 fathom = 6 ft, 1 ft = 12 in.,...
-
Consider Table 6-2. All duration estimates or estimated times are in days, and the network proceeds from Node 1 to Node 9. (Note that you can easily change this table to create multiple exercises.)...
-
Based on the information presented in the minicase, and the discussion in both Chapter 2 and this chapter, to what extent does Elon Musk fit the general charac- teristics of an entrepreneur and a...
-
The adjusted trial balance columns of the worksheet for Bramble Company are as follows. Bramble Company Worksheet (Partial) For the Month Ended April 30, 2022 Account Titles Adjusted Trial Balance...
-
Initially, each of the half-cells in Figure 19-21 contained a 100.0-mL sample of solution with an ion concentration of 1.000 M. The cell was operated as an electrolytic cell, with copper as the anode...
-
The electrolysis of Na 2 SO 4 (aq) is conducted in two separate half-cells joined by a salt bridge, as suggested by the cell diagram PtNa 2 SO 4 (aq)Na 2 SO 4 (aq)Pt. (a) In one experiment, the...
-
Do you believe that pay secrecy can ever really work in a business? Why, or why not?
-
Assume an organic compound has a partition coefficient between water and ethyl acetate equal to 8.12. If there are initially 7.10 grams of the compound dissolved in 75.0 mL of water, how many grams...
-
Berger Paint Pakistan limited produces three types of joint products, S ilver paint, G olden paint and D iamond paint. During March, 2020, the following information was recorded : Particulars Silver...
-
Choose an industry in which you are interested in working. How is that industry trending? What internal and external factors may affect the direction of the organization? For your initial post,...
-
show all work and Computations and if favorable or unfavorable. Standard Cost Data per 1 Unit Quantity Price Direct Labor 2 hrs $4.00/hr Actual Data: Units produced 20 20 Direct Labor 30 hrs; total...
-
4. A petroleum company is considering the expansion of its one unloading facility at its Australian refinery. Due to random variations in weather, loading delays, and other factors, ships arriving at...
-
With appropriate sketches, explain the changes that occur in the roll-pressure distribution if one of the rolls is idling, that is, power is shut off to that roll.
-
Velshi Printers has contracts to complete weekly supplements required by fortysix customers. For the year 2018, manufacturing overhead cost estimates total $600,000 for an annual production capacity...
-
What type of industry is likely to use a process cost system? Give some examples.
-
Your roommate asks your help in understanding the major steps in the flow of costs in a job order cost system. Identify the steps for your roommate.
-
There are two inventory control accounts in a job order system. Identify the control accounts and their subsidiary ledgers.
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


