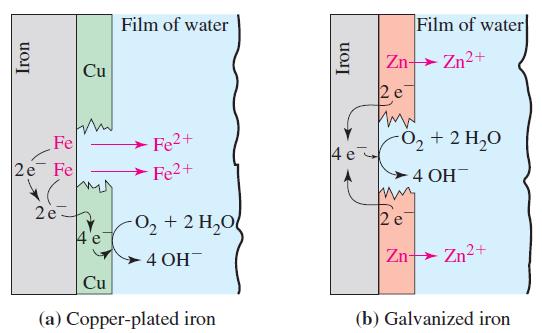
Initially, each of the half-cells in Figure 19-21 contained a 100.0-mL sample of solution with an ion
Question:
Initially, each of the half-cells in Figure 19-21 contained a 100.0-mL sample of solution with an ion concentration of 1.000 M. The cell was operated as an electrolytic cell, with copper as the anode and zinc as the cathode. A current of 0.500 A was used. Assume that the only electrode reactions occurring were those involving Cu/Cu2+ and Zn/Zn2+. Electrolysis was stopped after 10.00 h, and the cell was allowed to function as a voltaic cell. What was Ecell at that point?
Figure 19-21
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





