(A) Potassium crystallizes in the bcc structure. What is the length of the unit cell in this...
Question:
(A) Potassium crystallizes in the bcc structure. What is the length of the unit cell in this structure? Use the metallic radius of potassium given in Figure 9-11.
(B) Aluminum crystallizes in an fcc structure. Given that the atomic radius of Al is 143.1 pm, what is the volume of a unit cell?
Figure 9-11
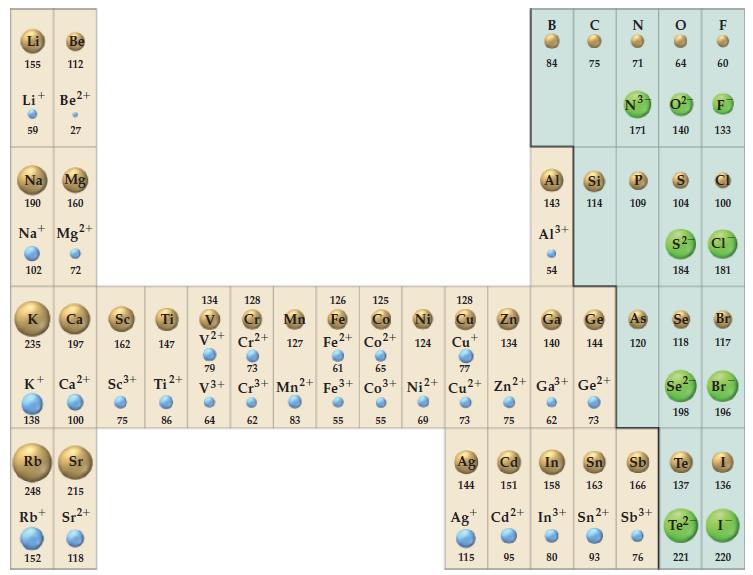
Transcribed Image Text:
Li 155 59 Na+ 102 Na Mg 190 160 K 235 Be 112 138 Be2+ 27 152 Mg2+ 72 K+ Ca²+ Sc³+ Ca Sc 197 162 100 Rb Sr 248 215 Rb Sr²+ 118 75 Ti 147 Tj ²+ 86 134 V²+ 79 V3+ 64 128 Cr²+ 73 3+ Mn²+ Cr 126 Ma Fe 127 Fe²+ Co²+ 124 62 ● 83 61 Fe³+ 55 125 55 128 Cu 69 Cu+ O 73 Ag 144 115 75 Cd 151 Ag Cd²+ B 84 Zn Ga 134 140 65 77 Co 3+ Ni2+ Cu2+ Zn²+ Ga³+ Ge²+ 95 Al 143 A1³+ 54 62 с In 3+ 75 80 Si 114 Ge 144 In Sn 158 163 73 Sn2+ 93 N 71 N3 171 109 120 Sb 166 Sb³+ 76 64 0² 140 $² 104 100 184 Se 118 Se² 198 60 Te2 133 221 L 181 Br 117 Br Te 137 136 196 I 220
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
A According to Figure 911 the metallic radius of potassium is 231 pm This means that the distance be...View the full answer

Answered By

Asd fgh
sadasmdna,smdna,smdna,msdn,masdn,masnd,masnd,m asd.as,dmas,dma.,sd as.dmas.,dma.,s ma.,sdm.,as mda.,smd.,asmd.,asmd.,asmd.,asm
5.00+
1+ Reviews
15+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Use the result of Practice Example 12-9A, the molar mass of K, and the Avogadro constant to calculate the density of potassium. (B) Use the result of Practice Example 12-9B, the molar mass of Al,...
-
(A) The ionic radius of Cs + is 167 pm. Use Figure 12-49 and information in Examples 12-9 and 12-11 to determine the edge length of the unit cell of CsCl. (B) Use the length of the unit cell of NaCl...
-
Aluminum metal crystallizes in a cubic close-packed structure [face-centered cubic cell, Figure 12.14(a)]. (a) How many aluminum atoms are in a unit cell? (b) What is the coordination number of each...
-
Provide an overview of the OS and the manufacturer What is the footprint for the selected OS? What security architecture was implemented? How many CVEs does this OS have?
-
Brown, located in Knoxville, contracted to buy sixty cases of Lovely Brand canned corn from Clark in Toledo at a contract price of $1,250. Pursuant to the contract, Clark selected and set aside sixty...
-
Crane Housing PLC has a current ratio of 2.5 and total current assets worth 5 million. Crane housing also has some long-term borrowings where the bank has stipulated that the current ratio of the...
-
Why are motorcoaches experiencing renewed growth as a transportation source?
-
Why does Harrahs system work so well compared to MIS efforts by other companies? Joseph, a 30-something New Yorker, recently went on a weekend trip to Atlantic City, New Jersey, where he hoped to...
-
Presented below are selected transactions on the book of Simonson Foundry. July 1, 2022 Bonds payable with a par value of $900,000, which is are dated January 1, 2022, are sold at $1,010,610, coupon...
-
At December 31, 2011, the records of Duo Corporation provided the following selected and incomplete data: Common stock (par $1; no changes during the year). Shares authorized, 5,000,000. Shares...
-
(A) The normal boiling point of isooctane (a gasoline component with a high octane rating) is 99.2 C, and its vap H is 35.76 kJ mol -1 . Because isooctane and water have nearly identical boiling...
-
In DNA the nucleic acid bases form hydrogen bonds between them, which are responsible for the formation of the double-stranded helix. Arrange the bases guanine and cytosine to give the maximum number...
-
Define the following terms: a. Multinational corporation b. Spot exchange rate c. Forward exchange rate d. Direct quote versus indirect quote e. Option f. LIBOR g. Euro
-
You have been recently hired to lead a Project to relocate your main Distribution Centre (DC) from Calgary, Alberta to St. John's, Newfoundland. As the Project Manager, try to complete a project plan...
-
Males Mean: 69.6 Standard Deviation: 11.3 For males, find P90, which is the pulse rate separating the bottom 90% from the top 10%.
-
Statistics Assignments Using Excel Assignment #4: Measures of Variability Part I Below are ACT composite scores from 20 randomly selected college students. 15 33 20 25 21 24 17 16 20 25 26 21 21 17...
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob ODonnell, a local merchant, to contribute the capital to form a partnership....
-
One of the main purposes of evaluation research is: a. reexamining previously collected data. b. monitoring and improving programs. c. generating rich descriptions of individual perspectives. d....
-
Complete the equation for each of the following reactions: a. CH3CH2CH2CO2H + PCl5 b. CH3(CH2)6CO2H + SOCl2 c. d. e. CH3(CH2)5CONH2 + LiAlH4 f. CH +KMnO4 CH3 co2NH+ heat CO,CH,CH, + LiAlH4
-
A regular deposit of $100 is made at the beginning of each year for 20 years. Simple interest is calculated at i% per year for the 20 years. At the end of the 20-year period, the total interest in...
-
Cash Flow Intuition A project has an initial cost of 1, has required return of R, and pays C annually for N years. a. Find C in terms of l and N such that the project has a payback period just equal...
-
MIRR Suppose the company in Problem 19 uses an 11 percent discount rate and an 8 percent reinvestment rate on all of its projects. Calculate the MIRR of the project using all three methods using...
-
Payback and NPV An investment under consideration has a payback of seven years and a cost of 5537.000. If the required return is 12 percent, what is the worst-case NPV the best-case NPV? Explain....
-
whether the following statements is TRUE or FALSE by providing a brief explanation . b) The Value at Risk of a first project is 8 and the Value at Risk of a second project is 5. The Value at Risk of...
-
Fill out a spreadsheet using the following information to perform a NPV analysis. Revenues in each of years 1-3 = $20,000 Year 0 initial investment = $40,000 Inventory level = $10,000 in year 1,...
-
A firm has $9.5 Billion debt outstanding, with a yield to maturity of 5.3% and a coupon rate of 4.6%. They have 148 million preferred shares outstanding, currently trading at $92.29. They also have...

Study smarter with the SolutionInn App


