A sample of NH 4 HS(s) is placed in a 2.58 L flask containing 0.100 mol NH
Question:
A sample of NH4HS(s) is placed in a 2.58 L flask containing 0.100 mol NH3(g). What will be the total gas pressure when equilibrium is established at 25 °C?
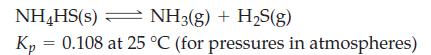
Transcribed Image Text:
NH4HS(s) NH3(g) + H₂S(g) Kp 0.108 at 25 °C (for pressures in atmospheres) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To determine the total gas pressure when equilibrium is established well first ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Sodium hydrogen carbonate (baking soda) decomposes at elevated temperatures and is one of the sources of CO 2 (g) when this compound is used in baking. What is the partial pressure of CO 2 (g)...
-
Weatherford International The oilfield services industry includes thousands of companies large and small that provide drilling, seismic testing, transportation, and a wide range of other services to...
-
(A) What will be the total gas pressure, in bar, if 12.5 g Ne is added to the mixture of gases described in Example 6-11 and the temperature is then raised to 55 C? (B) 2.0 L of O 2 (g) and 8.0 L of...
-
A project is proposed to design a database for shops selling dairy products. Each shop has a unique ID, name, address and owner. Different shops could be owned by the same owner. Each shop sells...
-
Charter Enterprises currently has $1 million in total assets and is totally equity financed. It is contemplating a change in its capital structure. Compute the amount of debt and equity that would be...
-
(a) What is meant by the phrase paper fortress? (b) How does maintaining a paper fortress aid the employer when the employee claims unfair treatment?
-
2. Describe how analyst projections of cyclical company profits compare with actual performance.What are the possible reasons for the deviation?
-
Given the following information, calculate after-tax cash flow for year 1. Assuming a sales price of $1,100,000, calculate the after-tax cash flow from the sale (dont forget the depreciation...
-
Exercise 6-11 (Part Level Submission) Sunland Excavating Inc. is purchasing a bulldozer. The equipment has a price of $96,700. The manufacturer has offered a payment plan that would allow Sunland to...
-
The following reaction is used in some self-contained breathing devices as a source of O 2 (g). Suppose that a sample of CO 2 (g) is added to an evacuated flask containing KO 2 (s) and equilibrium is...
-
One important reaction in the citric acid cycle is Write the equilibrium constant expression for the above reaction. Given that the concentrations of [citrate(aq)] = 0.00128 M, [aconitate(aq)] = 4.0...
-
Pepes Pizzeria has built its brand equity not only on the high quality of its food but on its social interaction with customers. They relate to their customers, says Pepes CEO Ken Berry. Kelly is a...
-
Nelsie Corporation has an outstanding 60-day 6% note receivable amounting to P 15,000 dated December of the ne year. The company is using the calendar year in preparing its financial statements. What...
-
Which resource is the bottleneck? What is the overall capacity of the orthopedist's office in patients/hour?
-
What are the comprehensive strategic implentation issues of Kmart with reference
-
1. Create both the written plan and the educational material to help African American women age 65+ control high blood pressure, take the special circumstances into consideration for the plan. 2. For...
-
Write down D & S equations for wireless phones; include two exogenous variables in each equation.
-
During a recent management meeting, Bruce Dunn, director of marketing, proposed that the company begin capitalizing its marketing expenditures as goodwill . In his words, "Marketing expenditures...
-
A Firm intends to invest some capital for a period of 15 years; the Firm's Management considers three Options, each consisting of purchasing a machinery of a specific brand, different for each...
-
As a preliminary to requesting budget estimates of sales, costs, and expenses for the fiscal year beginning January 1, 2011, the following tentative trial balance as of December 31, 2010, is prepared...
-
How often should standards be revised?
-
How are standards used in budgetary performance evaluation?
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...
-
Your portfolio has a beta of 1.17, a standard deviation of 14.3 percent, and an expected return of 12.5 percent. The market return is 11.3 percent and the risk-free rate is 3.1 percent. What is the...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...

Study smarter with the SolutionInn App


