A solution is prepared having these initial concentrations: [Fe 3+ ] = [Hg 2 2+ ] =
Question:
A solution is prepared having these initial concentrations: [Fe3+] = [Hg22+] = 0.5000 M; [Fe2+] = [Hg2+] = 0.03000 M. The following reaction occurs among the ions at 25 °C.
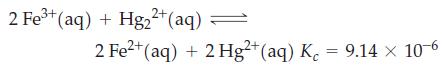
What will be the ion concentrations at equilibrium?
Transcribed Image Text:
2 Fe³+ (aq) + Hg2²+ (aq) 2 Fe²+ (aq) + 2 Hg2+ (aq) K = 9.14 x 10-6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Setting up the problem The balanced chemical equation for the reaction is 2 Fe3 aq Hg22 aq 2 Fe2 aq ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A solution is prepared from 0.150 mol of formic acid and enough water to make 0.425 L of solution. a. Determine the concentrations of H3O+ and HCOO in this solution. b. Determine the H3O+...
-
The following reaction occurs by a general-acid-catalyzed mechanism: Propose a mechanism for this reaction. CH2 CH3 HB+
-
The following reaction occurs by a mechanism involving general-base catalysis: Propose a mechanism for this reaction. base + CH,CH20H CH2OH
-
The annual revenues associated with several large apartment complexes are $300, $450, $425, $50, $75, and $150 for years 0, 1, 2, 3, 4, and 5, respectively. Determine the net cash flow and whether...
-
Security Data Company has outstanding 50,000 shares of common stock currently selling at $40 per share. The firm most recently had earnings available for common stockholders of $120,000, but it has...
-
Assume that in problem 11 the firm had purchased 80 October 1100 put option contracts on the S&P 500 Index listed in Table 165 on page 426, instead of selling the call options. If the S&P 500 Index...
-
5. An executive is reluctant to sell a high-performing business unit, arguing that the sale would dilute the companys ROIC to a level below the WACC and make the company value-destroying. Discuss.
-
Horace and Lee CPAs (H&L) is a medium-sized CPA firm that performs review and audit engagements for mostly privately held companies. H&L also has a tax group. H&L is considering accepting...
-
: What is the purpose of the general ledger? A. To record all financial transactions B. To prepare financial statements C. To track cash flows D. To calculate depreciation
-
Refer to the Integrative Example. A gaseous mixture is prepared containing 0.100 mol each of CH 4 (g), H 2 O(g), CO 2 (g), and H 2 (g) in a 5.00 L flask. Then the mixture is allowed to come to...
-
For the synthesis of ammonia at 500 K, N 2 (g) + 3 H 2 (g) 2 NH 3 (g), Kp = 9.06 x 10 -2 when the pressures are expressed in atmospheres. Assume that N 2 and H 2 are mixed in the mole ratio 1 : 3...
-
(Journal entries for several funds) The following transactions were incurred by the City of Mountain View. Record the journal entry (entries) necessary for each and identify the fund(s) used. If no...
-
SOUTHWEST AIRLINES: PROFILE OF A LEADER Airlines have faced economic difficulties with rising fuel costs and increased security standards. While many airlines have faced bankruptcy and corpo- rate...
-
a-1.If the required return is 11 percent, what is the profitability index for both projects? (Do not round intermediate calculations and round your answers to 3 decimal places, e.g., 32.161.) Project...
-
More info Mar. 1, 2024 Dec. 1, 2024 Dec. 31, 2024 Dec. 31, 2024 Jan. 1, 2025 Feb. 1, 2025 Mar. 1, 2025 Mar. 1, 2025 Borrowed $585,000 from Bartow Bank. The nine-year, 5% note requires payments due...
-
Describe the Leader(s) - Leadership Qualities/Style of Captain America in the movie The Avengers 1 (2012) Describe the actions that illustrate specific leadership characteristics and behaviors of...
-
During the current year, a company exchanged old equipment costing $ 6 4 , 0 0 0 with accumulated depreciation of $ 5 0 , 0 0 0 for a new truck. The new truck had a cash price of $ 8 0 , 0 0 0 and...
-
Victor Mineli, the new controller of Santorini Company, has reviewed the expected useful lives and salvage values of selected depreciable assets at the beginning of 2017. Here are his findings: All...
-
Arlington Merchants reported the following on its income statement for the fiscal years ending December 31, 2016 and 2015. 2016 2015 Sales $4,857,500 $4,752,900 Cost of goods sold 3,258,950 3,207,000...
-
An employee earns $40 per hour and 1.75 times that rate for all hours in excess of 40 hours per week. Assume that the employee worked 60 hours during the week, and that the gross pay prior to the...
-
Reaves Professional Services has three employees?a consultant, a computer programmer, and an administrator. The following payroll information is available for each employee: For the current pay...
-
In the following summary of data for a payroll period, some amounts have been intentionally omitted: (a) Calculate the amounts omitted in lines (1), (3), (8), and (12). (b) Journalize the entry to...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


