Refer to the Integrative Example. A gaseous mixture is prepared containing 0.100 mol each of CH 4
Question:
Refer to the Integrative Example. A gaseous mixture is prepared containing 0.100 mol each of CH4(g), H2O(g), CO2(g), and H2(g) in a 5.00 L flask. Then the mixture is allowed to come to equilibrium at 1000 K. What will be the equilibrium amount, in moles, of each gas?
Integrative Example
In the manufacture of ammonia, the chief source of hydrogen gas is the following reaction for the reforming of methane at high temperatures.
![]()
The following data are also given.
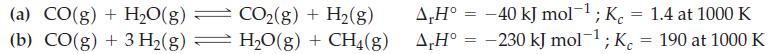
At 1000 K, 1.00 mol each of CH4 and H2O are allowed to come to equilibrium in a 10.0 L vessel. Calculate the number of moles of H2 present at equilibrium. Would the yield of H2 increase if the temperature were raised above 1000 K?
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





