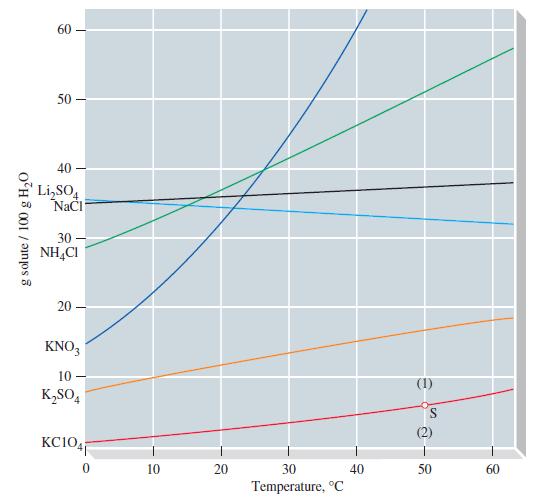
A solution prepared by dissolving 1.12 mol NH 4 Cl in 150.0 g H 2 O is
Question:
A solution prepared by dissolving 1.12 mol NH4Cl in 150.0 g H2O is brought to a temperature of 30 °C. Use Figure 14-10 to determine whether the solution is unsaturated or whether excess solute will crystallize.
Figure 14-10
Transcribed Image Text:
g solute/ 100 g H₂O 60 50- 40 Li₂SO4 NaCl 30 NH4Cl 20 KNO3 10- K₂SO4 KC1041 10 20 30 Temperature, °C 40 S 50 60
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
According to Figure 1410 the solubility of NH4Cl in water at 30 C ...View the full answer

Answered By

Divya Munir
I hold M.Sc and M.Phil degrees in mathematics from CCS University, India and also have a MS degree in information management from Asian institute of technology, Bangkok, Thailand. I have worked at a international school in Bangkok as a IT teacher. Presently, I am working from home as a online Math/Statistics tutor. I have more than 10 years of online tutoring experience. My students have always excelled in their studies.
4.90+
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A solution prepared by dissolving a 0.2541-g sample of electrolytic iron wire in acid was passed through a Jones reductor. The iron(II) in the resulting solution required a 36.76-mL titration....
-
2. Collars A and B move freely along the frictionless, vertical poles. Initially, collar A is at rest (VA = 0) and collar B has a constant velocity of 6 in/s downward. Collar A accelerates downward...
-
What will be the freezing temperature of a solution prepared by dissolving 4,000 mL of 1,2-ethanediol [(CH 2 CH 2 )(OH) 2 ; density= 1.12 g / mL] with 8 L of water? At what temperature will this...
-
Find each product. -3a+(4 + a)
-
Mokena, Inc. reported net income of $2.0 million in 2014. Depreciation for the year was $160,000, accounts receivable increased $350,000, and accounts payable increased $280,000. Compute net cash...
-
Bugg Properties expected EPS is \($2.00\) \($2.50\) and \($4.00\) for the next three years. Analysts expect that Bugg will pay dividends of \($1.00\) \($1.25\) and \($12.25\) for the three years. The...
-
New absence and holiday reporting procedures.
-
Venetian Corporation manufactures car stereos. It is a division of Berna Motors, which manufactures vehicles. Venetian sells car stereos to Berna, as well as to other vehicle manufacturers and retail...
-
How much sales are required to earn a target income of $206400 if total fixed costs are $258000 and the contribution margin ratio is 40%? O $774000 O $851400 $1161000 O $516000
-
An ideal liquid solution has two volatile components. In the vapor in equilibrium with the solution, the mole fractions of the components are (a) Both 0.50; (b) Equal, but not necessarily 0.50; (c)...
-
Of the following aqueous solutions, the one with the lowest freezing point is (a) 0.010 mol kg -1 MgSO 4 ; (b) 0.011 mol kg 1 NaCl; (c) 0.018 mol kg -1 CH 3 CH 2 OH; (d) 0.0080 mol kg 1 MgCl 2 .
-
The diagram shows two identical, charge-neutral, origin centered disks. One disk lies in the x-z plane. The other is tipped away from the first by an angle around the z-axis. The charge density of...
-
The Scantron Company makes bar-code scanners for major supermarkets. The sales staff estimates that the company will sell 500 units next year for 10,000 each. The production manager estimates that...
-
Determine the following: a. The stockholders equity of a company that has assets of \(\$ 625,000\) and liabilities of \(\$ 310,000\). b. The retained earnings of a company that has assets of \(\$...
-
You are the manager of internal audit for Do-It-All, Ltd., a large, diversified, decentralized manufacturing company. Over the past two years, the information systems function in Do-It-All has...
-
Following the example shown in (a) below, indicate the effects of the listed transactions on the assets, liabilities, and stockholders equity of John Dallmus, certified public accountant, a...
-
What effect does the ordering of a search tree have on the efficiency of the search? What effect does it have on the quality of the results? How would order affect the way that depth-first search or...
-
Given that v(0) = 2 and dv(0)/ dt = 4 , solve 10e ul) dt dt
-
The area of square PQRS is 100 ft2, and A, B, C, and D are the midpoints of the sides. Find the area of square ABCD. B A
-
Calculate the IRR (or IRRs) for the following project: For what range of discount rates does the project have positive-NPV? Co -3,000 +3,500 +4,000 -4,000
-
Consider the following two mutually exclusive projects: a. Calculate the NPV of each project for discount rates of 0, 10, and 20 percent. Plot these on a graph with NPV on the vertical axis and...
-
Mr. Cyrus Clops, the president of Giant Enterprises, has to make a choice between two possible investments: The opportunity cost of capital is 9 percent. Mr. Clops is tempted to take B, which has the...
-
As a staff accountant at a company that provides life insurance, the controller has asked you to prepare a report on the assumptions related to settlement costs needed to calculate the liability for...
-
OMEGA Hotel provides a type of rooms with a sale price of 50 euros. Its total fixed cost amounts to 100,000 euros. The variable cost per room was estimated at 25 euros. The dead point in rooms is:...
-
You are required to use a financial calculator or spreadsheet (Excel) to solve 10 problems (provided on page 5) on the applications of the time value of money. You are required to show the following...

Study smarter with the SolutionInn App


