(A) Start with DTBP at a pressure of 800.0 mmHg at 147 C. What will be the...
Question:
(A) Start with DTBP at a pressure of 800.0 mmHg at 147 °C. What will be the pressure of DTBP at t = 125 min, if t1/2 = 8.0 x 101 min? Because 125 min is not an exact multiple of the half-life, you must use equation (20.15). Can you see that the answer is between 200 and 400 mmHg?
Eq. 20.15
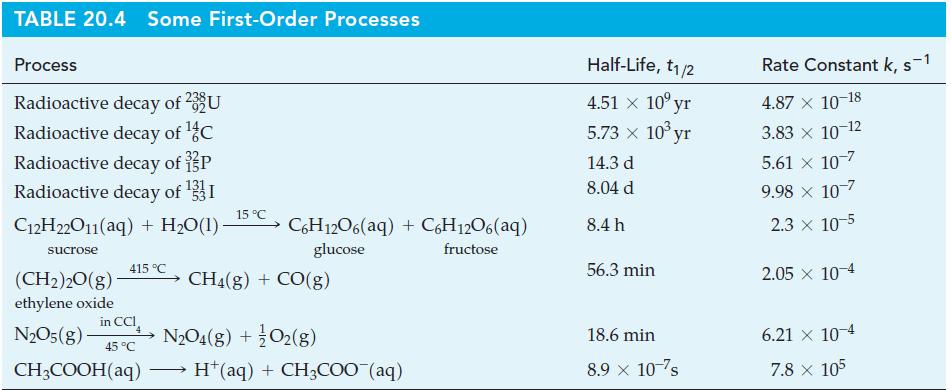
 (B) Use data from Table 20.4 to determine
(B) Use data from Table 20.4 to determine
(a) The partial pressure of ethylene oxide, and
(b) The total gas pressure after 30.0 h in a reaction vessel at 415 °C if the initial partial pressure of (CH2)2O(g) is 782 mmHg.
Table 20.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





