(A) The masses and percent isotopic abundances of the three naturally occurring isotopes of silicon are 28...
Question:
(A) The masses and percent isotopic abundances of the three naturally occurring isotopes of silicon are 28Si, 27.9769265325 u, 92.223%; 29Si, 28.976494700 u, 4.685%; 30Si, 29.973377017 u, 3.092%. Calculate the weighted-average atomic mass of silicon.
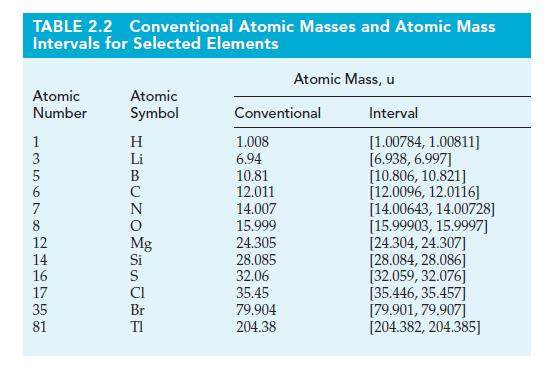
(B) Use data from Example 2-5 and the conventional atomic mass of Li (Table 2.2) to estimate the percent isotopic abundances of lithium-6 and lithium-7.
Example 2-5
(A) The two naturally occurring isotopes of boron, boron-10 and boron-11, have masses of 10.0129370 u and 11.0093054 u, respectively. Which of these two occurs in greater abundance?
(B) Indium has two naturally occurring isotopes and a weighted atomic mass of 114.818 u. One of the isotopes has a mass of 112.904058 u. Which of the following must be the second isotope: 111In, 112In, 114In, or 115In? Which of the two naturally occurring isotopes must be the more abundant?
Table 2.2
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





