(A) Use data from Table 7.2 to calculate the standard enthalpy of combustion of ethanol, C 2...
Question:
(A) Use data from Table 7.2 to calculate the standard enthalpy of combustion of ethanol, C2H5OH(l), at 298.15 K.
(B) Calculate the standard enthalpy of combustion at 298.15 K per mole of a gaseous fuel that contains C3H8 and C4H10 in the mole fractions 0.62 and 0.38, respectively.
Table 7.2
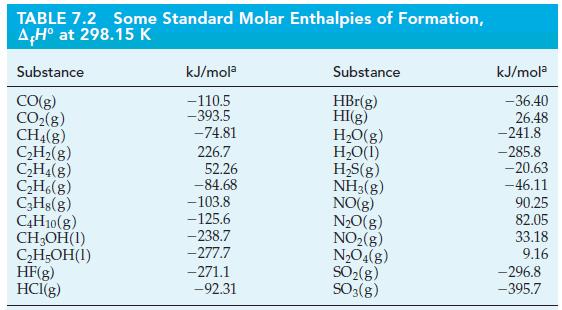
Transcribed Image Text:
TABLE 7.2 Some Standard Molar Enthalpies of Formation, A,Hº at 298.15 K Substance CO(g) CO₂(g) CH4(g) C₂H₂(g) C₂H4(8) C₂H6(g) C₂H8(g) C4H10(g) CH₂OH(1) C₂H5OH(1) HF(g) HCl(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(g) SO₂(g) SO3(g) kJ/mola - 36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
1 Answer to Part A The balanced equation for the combustion of ethanol is C2H5OHl 3O2g 2CO2g 3H2O...View the full answer

Answered By

Morgan Njeri
Very Versatile especially in expressing Ideas in writings.
Passionate on my technical knowledge delivery.
Able to multitask and able to perform under pressure by handling multiple challenges that require time sensitive solution.
Writting articles and video editing.
Revise written materials to meet personal standards and satisfy clients demand.
Help Online Students with their course work.
4.90+
12+ Reviews
38+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Ethanol (C2H5OH) has been proposed as an alternative fuel. Calculate the standard enthalpy of combustion per gram of liquid ethanol.
-
The standard enthalpy of combustion of solid urea (CO (NH2)2) is -632 kl mol-1 at 298 K and its standard molar entropy is 104.60 J K-1 mol-1, Calculate the standard Gibbs energy of formation of urea...
-
The molar heat capacity of ethane is represented in the temperature range 298 K to 400 K by the empirical expression Cp,m/ (J K-1 mol-1) = 14.73 + 0.1272(T/K). The corresponding expressions for C(s)...
-
What is the wavelength of light if its frequency is 1.009 106 Hz?
-
1. Which two short-term liquidity ratios measure how frequently a company collects its accounts? 2. What measure reflects the difference between current assets and current liabilities? 3. Which two...
-
Briefly describe four of the most frequently used methods for analyzing jobs.
-
What does cultural philosophy do?
-
Using the financial statement data provided in Exhibits 2, 3, and 4, match the companies with their industry. I Since oppornunities and constraints tend to be different across industries, companies...
-
12. During the Inter-war years, Federal Reserve keep raises interest rates, as an approach to make dollars more valuable. What is the result from this action? A. B. C. D. Maintain the value of the...
-
Minellan Ltd has extracted the following trial balance from its nominal ledger as at 31 March 2024: Additional information: (i) Inventory at 31 March 2024 was counted and valued at a cost of 181,000....
-
(A) The overall reaction that occurs in photosynthesis in plants is Determine the standard enthalpy of formation of glucose, C 6 H 12 O 6 (s), at 298.15 K. (B) A handbook lists the standard enthalpy...
-
(A) The standard enthalpy of formation for the amino acid leucine, C 6 H 13 O 2 N(s), is -637.3 kJ/mol. Write the chemical equation to which this value applies. (B) How is r H for the following...
-
The accompanying data are a subset of data from the report Great Jobs, Great Lives (Gallup-Purdue Index2015 Report, gallup.com/reports/197144/gallup -purdue-index-report-2015.aspx, retrieved April...
-
CASE STUDY Patient Name Valarie Ramirez Attending Paul F. Buckwalter, MD PATIENT INFORMATION DOB 08/04/1986 Allergies MAN 00-AA-006 Penicillin Other Information Past HX: AB x1 Valarie Ramirez arrives...
-
Petesy Corporation is preparing its Master Budget for 2019. Budget information is as follows: SalesProduction CostOperating Expenses 20191 st Quarter P280,000P192,000P64,000 2 nd Quarter 320,000...
-
A steady flow of 20 m3/s of moist air at TDB = 35iC, TWB = 25iC, 100 kPa (state 1) is dehumidified by first cooling it and condensing out moisture (state 2), then reheating it to 20iC and 50% R.H....
-
Question 37 Plantito Inc., produces potted plants. For next year, Pietro predicts that 45,000 units will be produced, with the following total costs: Direct materials Direct labor ? 80,000 Variable...
-
When you are to design a data transmission system, you have two key considerations to work with: data rate and distance, with emphasis placed on achieving the highest data rates over the longest...
-
Why are centerless grinders so popular in industry compared to center-type grinders?
-
For the next several days, take notes on your listening performance during at least a half-dozen situations in class, during social activities, and at work, if applicable. Referring to the traits of...
-
Ratio Computations and Effect of Transactions Presented below is information related to Leland Inc. (a) Compute the following ratios or relationships of Leland Inc. Assume that the ending account...
-
Current Liability Entries and Adjustments Described below are certain transactions of Edward son Corporation. The company uses the periodic inventory system. 1. On February 2, the corporation...
-
Liability Entries and Adjustments Listed below are selected transactions of Schultz Department Store for the current year ending December 31. 1. On December 5, the store received $500 from the...
-
Which do you prefer : a bank account that pays 4.5% per year (EAR) for three years or a) An account that pays 2.5% every 6 months for three years? b) An account that pays 6.5% every 18 months for...
-
10. Accounting firm XYZ provides audit tax and general accounting services, it estimates it will have 773 auditing 500 taxation, and 1,000 other service engagements. The company uses an ABC system...
-
Chapter 3: Adjusting Entries for Supplies Account and Unearned Revenue Account (Fee earned meaning Revenue) Q3-1. Western Company had $500 of store supplies available at the beginning of the current...

Study smarter with the SolutionInn App


