(A) Using data from Table 16.5, calculate the pH of 1.0 M Na 2 CO 3 ....
Question:
(A) Using data from Table 16.5, calculate the pH of 1.0 M Na2CO3.
(B) Using data from Table 16.5, calculate the pH of 0.500 M Na2SO3.
Table 16.5
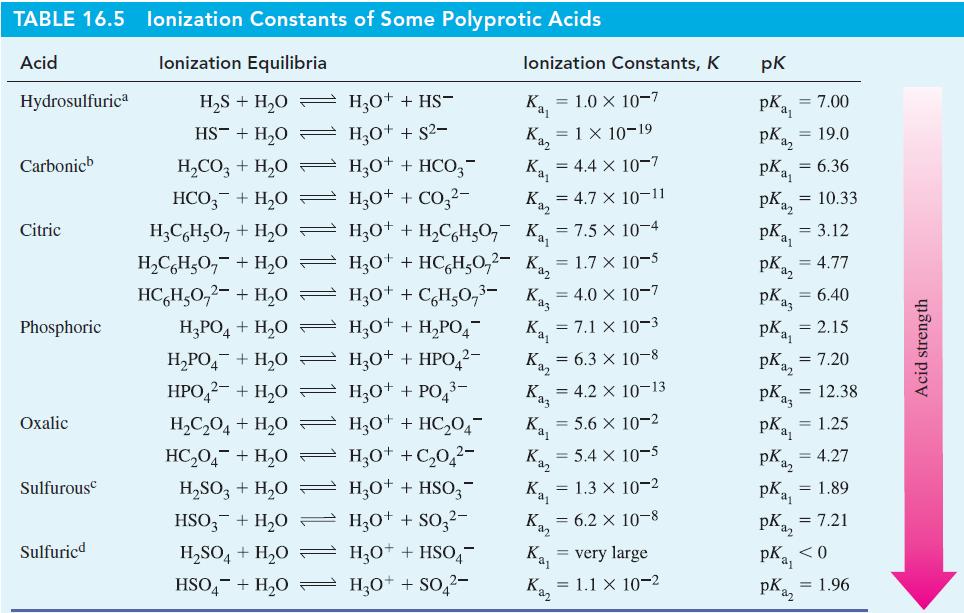
Transcribed Image Text:
TABLE 16.5 lonization Constants of Some Polyprotic Acids Acid lonization Equilibria Hydrosulfurica Carbonicb Citric Phosphoric Oxalic Sulfurous Sulfuricd H₂S + H₂O HS + H₂0 H₂CO3 + H₂O HCO3 + H₂0 H₂CH₂O7 + H₂0 H₂CH₂O + H₂0 HCHşO,2- + H,O − H₂PO4 + H₂0 H₂PO₂ + H₂O → HPO42- + H₂O = H₂C₂O4 + H₂0 HC₂04+H₂0 H₂SO3 + H₂O HSO3 + H₂0 H₂SO4+H₂O HSO4+H₂O → H₂O+ + HS- H₂O+ + S²- lonization Constants, K = 1.0 × 10-7 = 1 × 10-19 H₂O+ + HSO4- H₂O+ + SO4²- Kaz Ka₁ H₂0+ + HCO3- H₂O+ + CO3²- Kaz H3O+ + H₂CH₂O7 K₁₁ = 7.5 x 10-4 H3O+ + HC6H₂0,²-K₁₂ = 1.7 × 10-5 HO+ +C%HO, 3- H30+ + H₂PO4 H3O+ + HPO ²- H₂O+ + PO4³ 3- H30+ + HC₂04¯ H30++C₂04²- H3O+ + HSO3- H3O+ + SO3²- Kaz Ka₁ Kaz = 4.4 x 10-7 Kaj Kaz Kaz = 4.7 X 10-11 = 4.0 x 10-7 Kaş = 4.2 X 10-13 Ka₁ = 5.6 x 10-2 Kaz Kaj = 7.1 x 10-3 = 6.3 x 10-8 = 5.4 x 10-5 = 1.3 x 10-2 = 6.2 X 10-8 = very large = 1.1 x 10-2 pk pka pka₂ = 7.00 = 19.0 pkas pka₂ pk ₁₁ = 6.36 = 10.33 = 3.12 = 4.77 plaz PK₁3 = 6.40 pk₁₁ pKaz pKaz = 12.38 pka₁ = 1.25 pka₂ = 4.27 pk = 1.89 = 2.15 = 7.20 pk = 7.21 a₂ PK₁₁ <0 pKaz = 1.96 Acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
A To calculate the pH of 10 M Na2CO3 we need to consider the following reactions Na2CO3aq H2Ol 2 Naa...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Calculate the pH of a solution that contains the following analytical concentrations: (a) 0.225 M in H3PO4 and 0.414 M in NaH2PO4. (b) 0.0670 M in Na2SO3 and 0.0315 M in NaHSO3. (c) 0.640 M in...
-
Calculate the pH of a 1 L solution containing (a) 10 mL of 5 M NaOH, (b) 10 mL of 100 mM glycine and 20 mL of 5 M HCl, and (c) 10 mL of 2 M acetic acid and 5 g of sodium acetate (formula weight 82 g ...
-
Using the data in Problem 14-10, calculate the pH of a 1.00 10-2 M NaOH solution at 508C.
-
The coordinates of points A and B are given below: Easting Northing Height (meter) (meter) (meter) 41.676 66.446 225.973 127.066 31.063 185.401 Point A B What is the bearing of AB (from A to B)?...
-
What is corporate governance? How has the Sarbanes-Oxley Act of 2002 affected it? Explain.
-
a. Estimate the potential capability of the process. b. Estimate the actual process capability. c. How much improvement could be made in process performance if the mean could be centered at the...
-
Using the data on the two part numbers given, provide a comprehensive evaluation of the ordering policies. Compare the present annual average cost with the cost of using a system such as EOQ, and...
-
What interest rates should be used in determining the amount of interest to be capitalized? How should the amount of interest to be capitalized be determined?
-
Lavage Rapide is a Canadian company that owns and operates a large automatic car wash facility near Montreal. The following table provides estimates concerning the companys costs: Fixed Cost per...
-
(A) A 20.00 mL sample of 0.150 M HF solution is titrated with 0.250 M NaOH. Calculate (a) The initial pH and the pH when neutralization is (b) 25.0%, (c) 50.0%, and (d) 100.0% complete. (B) For the...
-
What concentration of ammonia, [NH 3 ], should be present in a solution with [NH 4 + ] = 0.732 M to produce a buffer solution with pH = 9.12? For NH 3 , K b = 1.8 x 10 -5 .
-
Will direct response communications play a more significant role in the marketing communications mix in the future? Through secondary research, identify those factors that will encourage or...
-
"The initial speed with which a ball is thrown is doubled, with the angle of projection fixed. Is the maximum height to which the ball rises doubled?" Now, let's say you are also allowed to change...
-
Wally Working Co. emiti bonos con una tasa de inters nominal (contratada) de 15%, por un valor ominal de $80,000, con un vencimiento de 5 anios. Cuando emiti los bonos, la tasa de inters del mercado...
-
Using the Central Limit Theorem. In Exercises 5-8, assume that the amounts of weight that male college students gain during their freshman year are normally distributed with a mean of 1.2 kg and a...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Please help Calculating NPV and IRR Businesses use NPV and IRR to determine whether a project will add - value for shareholders. After watching the CFA Level I Corporate Finance video, answer the...
-
You are a loan officer for White Sands Bank of Taos. Paul Jason, president of P. Jason Corporation, has just left your office. He is interested in an 8-year loan to expand the company's operations....
-
Burberrys competitive advantage is through its differentiation strategy. What risk should Burberry remain aware of?
-
Summit Co., a furniture wholesaler, sells merchandise to Bitone Co. on account, $23,400, terms 2/10, n/30. The cost of the merchandise sold is $14,000. Summit Co. issues a credit memo for $4,400 for...
-
Based on the data presented in Exercise 6-26, journalize Bitone Co.s entries for (a) The purchase, (b) The return of the merchandise for credit, and (c) The payment of the invoice within the discount...
-
What is the normal balance of the following accounts? (a) Cost of Merchandise Sold, (b) Delivery Expense, (c) Merchandise Inventory, (d) Sales, (e) Sales Discounts, (f) Sales Returns and Allowances,...
-
Construction of consumer price index number for the given goods and services. Item Weight in % Base period price Current period price Food 35 150 145 Fuel 10 25 23 Cloth 20 75 65 Rent 15 30 30 Misc....
-
Gammaro Corporation has found that 80% of its sales in any given month are credit sales, while the remainder are cash sales of the credit sales, Gammaro Corporation has experienced the following...
-
Swifty Company estimates that 2022 sales will be $43,200 in quarter 1,$51,840 in quarter 2 , and $62,640 in quarter 3 , Cost of goods sold is 50% of sales. Management desires to have ending...

Study smarter with the SolutionInn App


