(A) What is E cell for the reaction in which Cl 2 (g) oxidizes Fe 2+ (aq)...
Question:
(A) What is E°cell for the reaction in which Cl2(g) oxidizes Fe2+(aq) to Fe3+(aq)?
![]()
(B) Use data from Table 19.1 to determine E°cell for the redox reaction in which Fe2+(aq) is oxidized to Fe3+(aq) by MnO4-(aq) in acidic solution.
Table 19.1
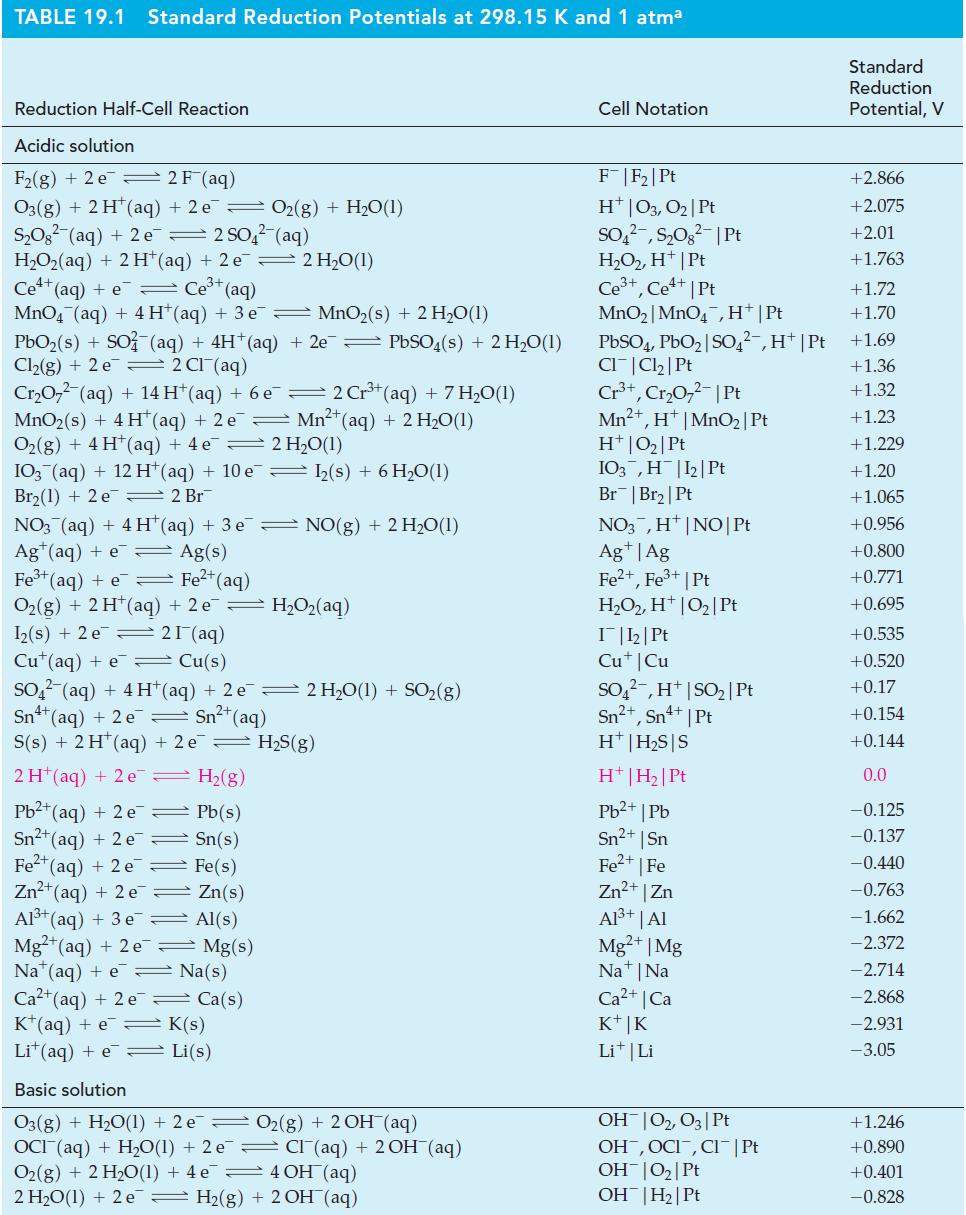
Transcribed Image Text:
3+ 2 Fe2+ (aq) + Cl₂(g) — 2 Fe³+ (aq) + 2 CI¯ (aq) Ecell ? =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
The standard cell potential E for a given redox reaction can be calculated using the standar...View the full answer

Answered By

Payal Mittal
I specialize in finance and accounts.You can ask any question related to til undergradution.Organizational behaviour and HRM are my favourites for you can always relate to them and is an art with practical knowledge base.
4.90+
226+ Reviews
778+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Consider gravity loading only under complete lateral restraint in flat strata with properties given in Table 1.3. Vertical stress at the top of the geologic column given in Table 1.3 is 6.9 MPa....
-
In one type of Breathalyzer (alcohol meter), the quantity of ethanol in a sample is related to the amount of electric current produced by an ethanoloxygen fuel cell. Use data from Table 19.1 and...
-
(A) Use data from Table 19.1 to predict the probable products when Pt electrodes are used in the electrolysis of KI(aq). Table 19.1 (B) In the electrolysis of AgNO 3 (aq), what are the expected...
-
In Exercises 1114, graph each equation in a rectangular coordinate system. If two functions are indicated, graph both in the same system. Then use your graphs to identify each relations domain and...
-
Identify each of the following decision as most directly related to (a) Cash flow management (b) Choice of inventory system (c) Foreign merchandising transactions: 1. Determination of the amount of...
-
Canidae Corporation, based in San Luis Obispo, California, is a producer of pet food. One of its products is Felidae cat food. The Chicken and Rice Cat and Kitten Formula is a dry cat food that comes...
-
Appreciate why nations trade. LO.1
-
Exhibit 1: Prices and projected annual sales volumes for Sony PlayStation 3 and Microsoft Xbox 360 Elite Exhibit 2: Sony PlayStation 3 Production Costs per unit in dollars Exhibit 3: Microsoft Xbox...
-
Credit collections are 5% two months following the sale, 43% in the month following the sale, and 50% in the month of the sale. The remaining 2% is expected to be uncollectable. what is the total...
-
A new battery system currently under study for possible use in electric vehicles is the zincchlorine battery. The overall reaction producing electricity in this cell is Zn(s) + Cl 2 (g) ZnCl 2 (aq)....
-
(A) The cell diagram for an electrochemical cell is written as Write the equations for the half-cell reactions that occur at the electrodes. Balance the overall cell reaction. (B) The cell diagram...
-
A 40-lb box slides from rest down the smooth ramp onto the surface of a 20-lb cart. Determine the speed of the box at the instant it stops sliding on the cart. If someone ties the cart to the ramp at...
-
3. Given the continuous beam shown below, which span or spans should be loaded with a uniform distributed load to produce a maximum moment at support B? (5 points) SPAN 1 SPAN 2 SPAN 3 A B D 20 ft...
-
Complete the following writing assignment: Analyze the attached 10_pages. Write_about them, summarize what you read, and connect it to personal experiences. CHAPTER 8 Anxiety Disorders DAVID P....
-
As a manager of an airline company you want to learn the average weight of luggages checked in on a flight. From a sample of 1 6 luggages, you find the average to be 2 6 kg and the standard deviation...
-
What is the Manufacturing Cycle Efficiency? 11. Use High-Low to find the fixed and variable costs. Machine Month Costs Hours 12345678 $1,730,890 15,820 $1,753,860 13,980 $1,562,890 11,550 4...
-
Jimmy Padilla purchased a gravel pit in the current year for $944,232 and estimates that there will be a residual value in the land of $36,404 once resource extraction is complete. He estimates that...
-
Assume Doltron Co. paid $18 million to purchase Bailey Industries. Assume further that Bailey Industries had the following summarized data at the time of the Doltron Co. acquisition (amounts in...
-
X-1 Find the domain of the function f(x) : x 1 2 - O (-00, -1) U (-1, ) O (-00, 1) U (1, ) O -00, -1) U (-1, 1) U (1, 0) O (- 1, 1)
-
(a) What is a lease agreement? (b) What are the two most common types of leases? (c) Distinguish between the two types of leases.
-
In general, what are the requirements for the financial statement presentation of long-term liabilities?
-
Laura Hiatt is discussing the advantages of the effective interest method of bond amortization with her accounting staff. What do you think Laura is saying?
-
Summarize in your own words Sharps, Treynors, and Jensens Measures for assessing portfolio performance with respect to risk. Assess the portfolio performance of mutual fund VDIGX taking into...
-
Question 1 Slat and Company have recently set up a business which will manufacture and sell a furniture component, the F12 On the 19 August 2021, the company issued 85,000 of share capital for cash....
-
The following is Addison Corporations contribution format income statements for last month. The company has no beginning or ending inventories. A total of 10,000 units were produced and sold last...

Study smarter with the SolutionInn App


