In one type of Breathalyzer (alcohol meter), the quantity of ethanol in a sample is related to
Question:
In one type of Breathalyzer (alcohol meter), the quantity of ethanol in a sample is related to the amount of electric current produced by an ethanol–oxygen fuel cell. Use data from Table 19.1 and Appendix D to determine
(a) E°cell and
(b) E° for the reduction of CO2(g) to CH3CH2OH(g).
Table 19.1
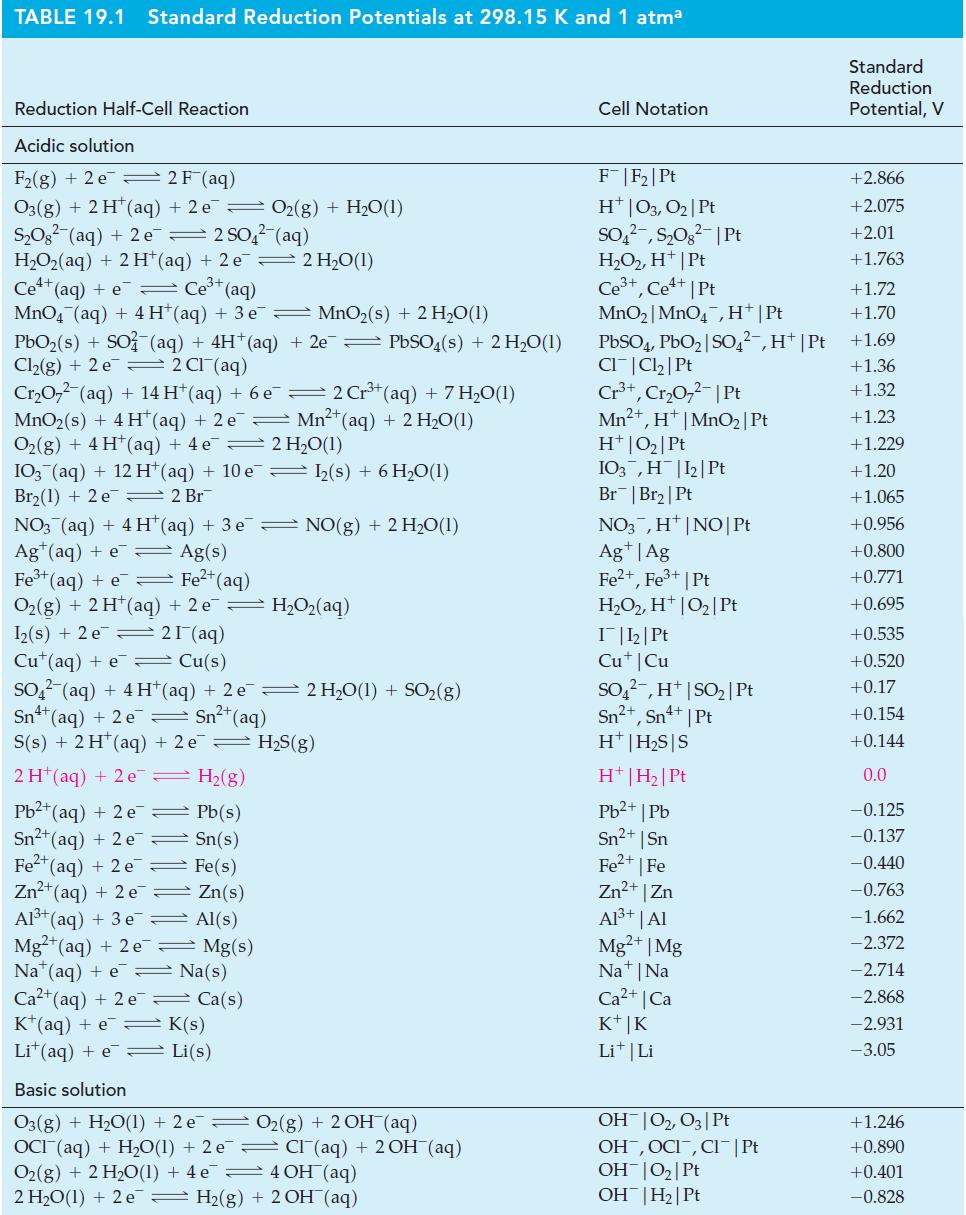
Transcribed Image Text:
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma
Reduction Half-Cell Reaction
Acidic solution
F₂(g) + 2 e 2F (aq)
O3(g) + 2 H¹ (aq) + 2 e O₂(g) + H₂O(1)
S₂O² (aq) + 2 e
2 SO4²(aq)
H,Oz(aq) + 2H*(aq) +2e <2H,O(1)
Ce+ (aq) + e Ce³+ (aq)
MnO4 (aq) + 4H*(aq) + 3 e¯ ⇒ MnO₂(s) + 2 H₂O(1)
PbO₂ (s) + SO² (aq) + 4H+ (aq) + 2e¯ — PbSO(s) + 2 H₂O(1)
Cl₂(g) + 2 e 2 CI (aq)
Cr₂O7² (aq) + 14 H*(aq) + 6 e¯ — 2 Cr³+ (aq) + 7 H₂O(1)
MnO2 (s) + 4H* (aq) + 2 e Mn²+ (aq) + 2 H₂O(1)
O₂(g) + 4H+ (aq) + 4e
2 H₂O(1)
IO3 (aq) + 12 H*(aq) + 10 e
Br₂(1) 2 e 2 Br
NO3(aq) + 4H(aq) + 3 e¯¯ — NO(g) + 2 H₂O(1)
Ag¹(aq) + e
Ag(s)
Fe³+ (aq) + e
Fe²+ (aq)
Oz(g) +2H*(aq) +2e
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma
Reduction Half-Cell Reaction
Acidic solution
F₂(g) + 2 e 2F (aq)
O3(g) + 2 H¹ (aq) + 2 e O₂(g) + H₂O(1)
S₂O² (aq) + 2 e
2 SO4²(aq)
H,Oz(aq) + 2H*(aq) +2e <2H,O(1)
Ce+ (aq) + e Ce³+ (aq)
MnO4 (aq) + 4H*(aq) + 3 e¯ ⇒ MnO₂(s) + 2 H₂O(1)
PbO₂ (s) + SO² (aq) + 4H+ (aq) + 2e¯ — PbSO(s) + 2 H₂O(1)
Cl₂(g) + 2 e 2 CI (aq)
Cr₂O7² (aq) + 14 H*(aq) + 6 e¯ — 2 Cr³+ (aq) + 7 H₂O(1)
MnO2 (s) + 4H* (aq) + 2 e Mn²+ (aq) + 2 H₂O(1)
O₂(g) + 4H+ (aq) + 4e
2 H₂O(1)
IO3 (aq) + 12 H*(aq) + 10 e
Br₂(1) 2 e 2 Br
NO3(aq) + 4H(aq) + 3 e¯¯ — NO(g) + 2 H₂O(1)
Ag¹(aq) + e
Ag(s)
Fe³+ (aq) + e
Fe²+ (aq)
Oz(g) +2H*(aq) +2e
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A social worker notices that some of her most anxious clients often come to see her with coffee mugs in hand. Wondering if their anxiousness (X) is related to their amount of caffeine consumption...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
In the light of what you know about IHRM, how easy do you think it is to manage a global employer brand? What issues would an IHRM professional expect to have to manage? To answer this, analyse the...
-
Rianne Company produces a light fixture with the following unit cost: Direct materials ...... $2 Direct labor ......... 1 Variable overhead ..... 3 Fixed overhead ....... 2 Unit cost ......... $8 The...
-
Consider Figure 5-7b. Assuming that the empty rows in the leaves of this index show space where new records can be stored, explain where the record for Sooners would be stored. Where would the record...
-
How will the debt-management plan work? What debts can be included in the plan, and will you get regular reports on your accounts?
-
The ledger of Jung Company includes the following accounts. Explain why each account may require adjustment. (a) Prepaid Insurance. (b) Depreciation Expense. (c) Unearned Service Revenue. (d)...
-
Mindy sold her interest in a partnership for $20,000 cash when her basis was 55.000 She was relieved of her $25,000 share of partnership liabilities. What is Mindy's recognised gain from the sale of...
-
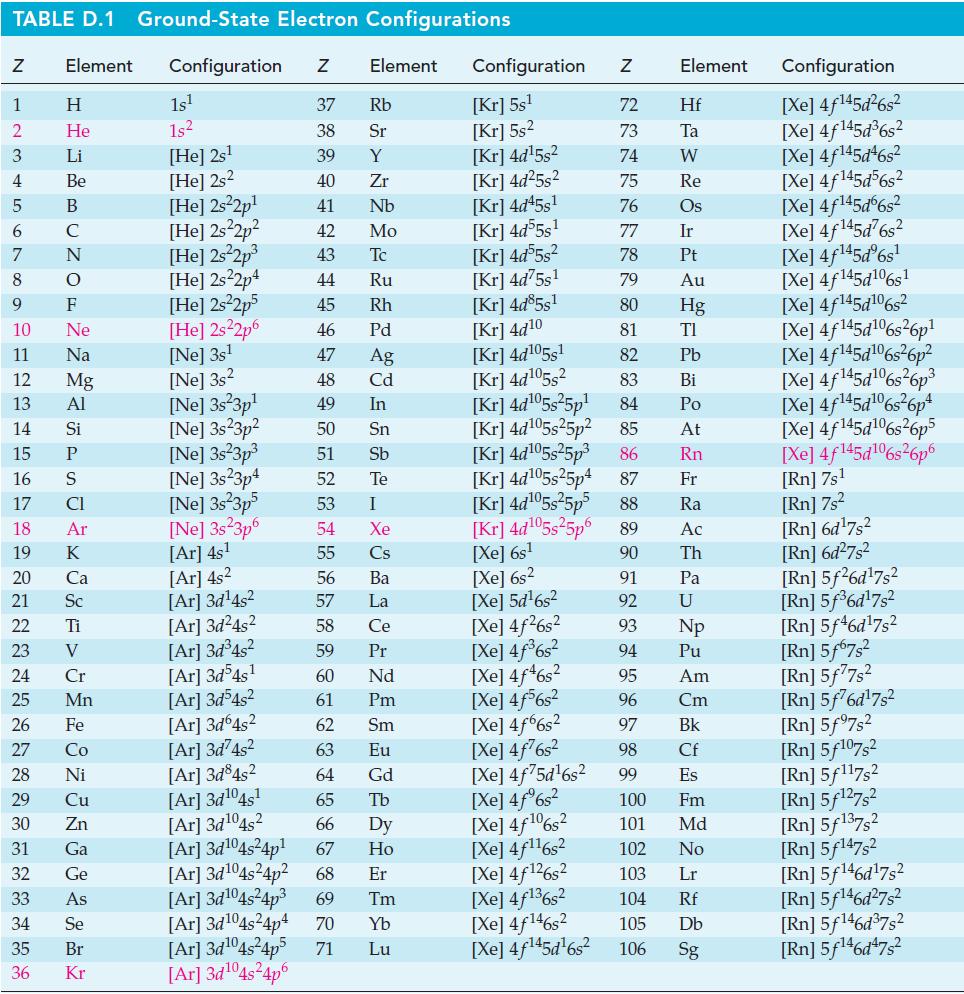
Show that for some fuel cells the efficiency value, e = r G/ r H,can have a value greater than 1.00. Can you identify one such reaction? Use data from Appendix d. TABLE D.1 Ground-State Electron...
-
Isobutylene (2-methylpropene) can be polymerized by treating it with liquid HF as shown in Fig. P5.53. A small amount of tert-butyl fluoride is formed in the reaction. Suggest a curved-arrow...
-
The Wide World of Fluids article titled "A Sailing Ship without Sails,". Determine the magnitude of the total force developed by the two rotating cylinders on the Flettner "rotor-ship" due to the...
-
Section Three Answer the questions below 1.While pulling out of her driveway, Bethany becomes distracted by a bee and strikes Melanie, who is riding past on a bicycle. Bethany suffers serious injury...
-
A __________ is a schedule periodic check of a specific process behavior. Question 1Answer A. Widget B. Dashboard C. Monitor D. Process ID
-
1. Was VAAF contractually obligated to pay Chad for refraining from smoking? 2. Was there consideration to support its promise to pay $500? 3. Are there other facts you need to know to make that...
-
Presented here are the comparative balance sheets of Hames Incorporated at December 31, 2023 and 2022. Sales for the year ended December 31, 2023, totaled $1,700,000.%0D%0A%0D%0AHAMES...
-
McDonald's conducts operations worldwide and is managed in two primary geographic segments: US, and International Operated Markets, which is comprised of Australia, Canada, France, Germany, Italy,...
-
Given the function y = 5x, complete the following schedule and plot the curve. x|-4-20
-
Solve the relation Exz:Solve therelation ne %3D
-
Sam Farr is the president, founder, and majority owner of Galena Medical Corporation, an emerging medical technology products company. Galena is in dire need of additional capital to keep operating...
-
Numerous articles have been written that identify early warning signs that you might be getting into trouble with your personal debt load. You can find many good articles on this topic on the Web....
-
Nordham Corporations trial balance at December 31, 2010, is presented below. All 2010 transactions have been recorded except for the items described below and on the next page. Unrecorded...
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...

Study smarter with the SolutionInn App


