Show that for some fuel cells the efficiency value, e = r G/ r H,can have
Question:
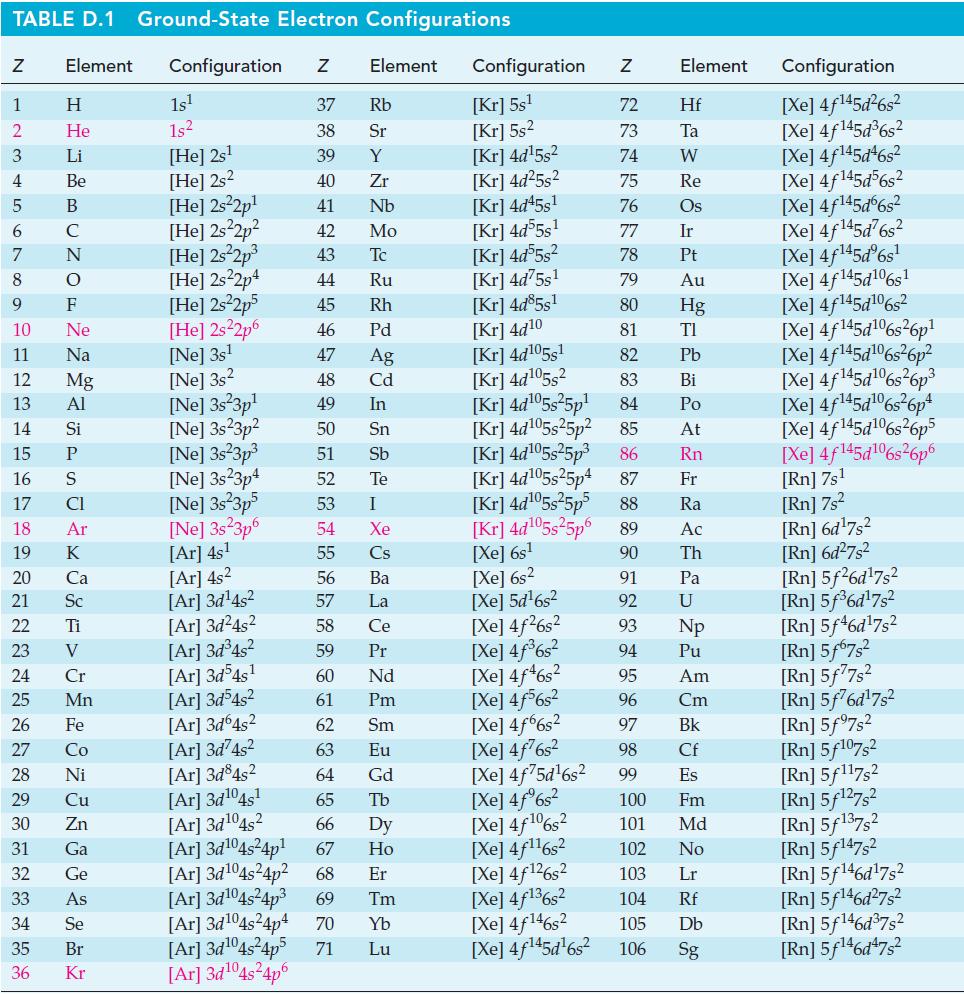
Show that for some fuel cells the efficiency value, e = ΔrG°/ΔrH°,can have a value greater than 1.00. Can you identify one such reaction? Use data from Appendix d.
Transcribed Image Text:
TABLE D.1 Ground-State Electron Configurations Element Configuration Z Z 1 2 3 4 5 6 7 8 9 HIG&LUZONES JY SE > 0 ≤ 2 3 2 3 5 3 3 2 2 5 2 He 10 11 12 13 14 15 16 17 18 19 20 21 Sc 22 23 24 25 Mn Mg 26 27 28 Ni 29 30 31 32 33 34 35 36 Zn Ga Ge 1s¹ 1s² [He] 2s¹ [He] 2s2 [He] 2s²2p¹ [He] 2s²2p² [He] 2s²2p³ [He] 2s22p4 [He] 2s²2p5 [HE] 2s²2p6 [Ne] 3s¹ [Ne] 3s2 [Ne] 3s23p¹ [Ne] 3s23p² 37 Rb 38 Sr 39 Y 40 Zr 41 Nb 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 Sb 52 Te 53 54 55 [Ar] 4s² 56 [Ar]3d¹4s² 57 [Ar]3d²4s² 58 [Ar]3d³4s² 59 [Ar]3d54s¹ 60 61 [Ar]3d³4s² [Ar]3d64s² 62 [Ar]3d²4s² 63 [Ar] 3d84s² 64 [Ar]3d¹04s¹ 65 [Ar]3d¹04s2 [Ar]3d¹04s²4p¹ [Ar]3d¹04s²4p² [Ar]3d¹04s²4p³ 69 Tm 66 Dy 67 Ho 68 Er [Ar]3d¹04s²4p4 70 [Ar]3d¹04s²4p5 71 Yb Lu [Ar] 3d¹04s²4p6 [Ne] 3s²3p³ [Ne] 3s23p4 [Ne] 3s²3p5 Element [Ne] 3s 3p6 [Ar] 4s¹ I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Configuration Z [Kr] 5s¹ [Kr] 5s² [Kr] 4d¹5s² [Kr] 4d²5s² [Kr] 4d45s¹ [Kr] 4d55s¹ [kr] 4d55s² [Kr] 4d75s¹ [Kr] 4d85s1 [Kr] 4d10 [Kr] 4d105s1 [Kr] 4d¹05s² [Kr] 4d¹05s²5p¹ [Kr] 4d¹05s25p² [Kr] 4d¹05s²5p³ [KR] 4d¹05s25p4 [Xe] 6s² [Xe] 5d¹6s² [Xe] 4f²6s² [Xe] 4f³6s² [Xe] 4f46s2 [Xe] 4f6s2 [Xe] 4f6s2 [Xe] 4f²6s² [Xe] 4f75d¹6s² [Xe] 4f%s2 [Xe] 4f106s2 [Xe] 4f¹¹6s² NRNKERKR [Xe] 4f126s2 [Xe] 4f136s2 [Xe] 4f146s2 [Xe] 4f¹45d¹6s² 72 Hf 73 Ta W 74 75 Re 76 Os 77 Element 78 79 80 81 82 83 84 85 86 87 Fr [Kr] 4d¹05s²5p5 88 Ra [Kr] 4d¹05s²5p6 89 Ac [Xe] 6s¹ 90 Th 91 92 93 94 95 96 97 98 99 Ir Pt Au Hg TI Pb Bi Po At Rn Pa U Np Pu Am Cm Bk Cf Es 100 Fm 101 Md 102 No 103 Lr 104 Rf 105 Db 106 Sg Configuration [Xe] 4f¹45d²6s² [Xe] 4f145d³6s² [Xe] 4f145d46s2 [Xe] 4f145d56s2 [Xe] 4f145d6s2 [Xe] 4f¹45d²6s² [Xe] 4f¹45dº6s¹ [Xe] 4f145d106s1 [Xe] 4f145d106s2 [Xe] 4f145d6s26p* [Xe] 4f145d106s36p? [Xe] 4f145d16s?6p3 [Xe] 4f145d6s®6p* [Xe] 4f145d16s26p5 [Xe] 4f145d106s26p6 [Rn] 7s¹ [Rn] 7s² [Rn] 6d¹7s² [Rn] 6d²7s² [Rn] 5f26d¹7s² [Rn] 5f³6d¹7s² [Rn] 5f46d¹7s2 [Rn] 5f67s² [Rn] 5f77s² [Rn] 5f76d¹7s² [Rn] 5f97s2 [Rn] 5f107,2 [Rn] 5f117s2 [Rn] 5f¹27s² [Rn] 5f137,2 [Rn] 5f147s2 Rn] 5f¹46d¹7s² [Rn] 5f¹46d²7s² [Rn] 5f¹46d³7s2 [Rn] 5f¹6d¹7s²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
o show that the efficiency of a fuel cell can be greater than 100 I can use the following equation e ...View the full answer

Answered By

Hassan Ali
I am an electrical engineer with Master in Management (Engineering). I have been teaching for more than 10years and still helping a a lot of students online and in person. In addition to that, I not only have theoretical experience but also have practical experience by working on different managerial positions in different companies. Now I am running my own company successfully which I launched in 2019. I can provide complete guidance in the following fields. System engineering management, research and lab reports, power transmission, utilisation and distribution, generators and motors, organizational behaviour, essay writing, general management, digital system design, control system, business and leadership.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In Exercises 60-64, use the diagram at the right. Name all the rays that contain point C. P Q A ( C E ' D
-
Use the information given in the diagram to write a plan for proving that 1 2. A 1 F B E C 2 D
-
Construct solid-liquid and solid-vapour phase boundaries for iodine I 2 given the following: Density of solid I 2 = 4.93 g cm 3 Density of liquid I 2 = 3.96 g cm 3 Enthalpy of fusion = 14.73 kJ/mol...
-
How will you sort 1 PB numbers? 1 PB = 1000 TB.
-
Hetrick Dentistry Services operates in a large metropolitan area. Currently, Hetrick has its own dental laboratory to produce porcelain and gold crowns. The unit costs to produce the crowns are as...
-
List three common situations that suggest that relations be denormalized before database implementation.
-
If youre paying a lot more, you may be the one whos being set up.
-
Suppose Sparrow Sporting Goods Company reported the following data at March 31, 2012, with amounts in thousands: Use these data to prepare Sparrow Sporting Goods Company's income statement for the...
-
The First National Bank is offering a 3-year certificate of deposit (CD) at 4% interest compounded quarterly; Second National Bank is offering a 3 year CD at 5% interest compounded annually. (Round...
-
It is sometimes possible to separate two metal ions through electrolysis. One ion is reduced to the free metal at the cathode, and the other remains in solution. In which of these cases would you...
-
In one type of Breathalyzer (alcohol meter), the quantity of ethanol in a sample is related to the amount of electric current produced by an ethanoloxygen fuel cell. Use data from Table 19.1 and...
-
Find the volume charge density and surface charge density which must be placed in and on a sphere of radius R to produce a field inside the sphere of: E = 18 + 1/8 (1 - 138 - 1/0 2. Vo Vo x ) R3...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 C. Determine the magnitude and direction of the electric field along the axis of the rod at a point 32.0 cm from its center....
-
Hello need help with this problem. The transactions relating to the formation of Blue Company Stores Incorporated, and its first month of operations follow. a. The firm was organized and the...
-
At the beginning of the year, the net assets of Shannon Company were $492,600. The only transactions affecting stockholders equity during the year were net income of $70,200 and dividends of $15,400....
-
The claim is that smokers have a mean cotinine level greater than the level of 2.84 ng/mL found for nonsmokers. (Cotinine is used as a biomarker for exposure to nicotine.) The sample size is n = 739...
-
Given the function y = 8 - 2x, complete the following schedule and plot the curve. L-4-2024
-
CRUZ, INC. Comparative Balance Sheets December 31, 2015 CRUZ, INC. Income Statement For Year Ended December 31, 2015 Required Use the indirect method to prepare the cash provided or used from...
-
Bond or debt securities pay a stated rate of interest. This rate of interest is dependent on the risk associated with the investment. Moodys Investment Service provides ratings for companies that...
-
On January 1, 2008, Carlin Corporation issued $2,400,000 of 5-year, 8% bonds at 95; the bonds pay interest semiannually on July 1 and January 1. By January 1, 2010, the market rate of interest for...
-
Joe Penner, president of Penner Corporation, is considering the issuance of bonds to finance an expansion of his business. He has asked you to (1) Discuss the advantages of bonds over common stock...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


