(A) What is the mass of 2.35 x 10 24 atoms of Cu? (B) How many lead-206...
Question:
(A) What is the mass of 2.35 x 1024 atoms of Cu?
(B) How many lead-206 atoms are present in a 22.6 g sample of lead metal? See Figure 2-16.
Figure 2-16
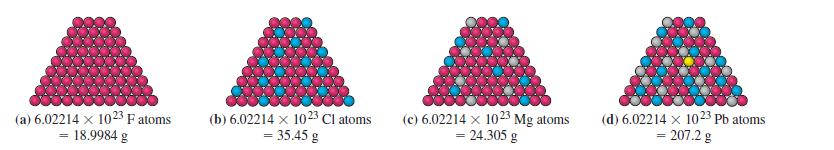
Transcribed Image Text:
(a) 6.02214 x 1023 F atoms = 18.9984 g (b) 6.02214 x 1023 Cl atoms = 35.45 g (c) 6.02214 x 1023 Mg atoms = 24.305 g (d) 6.02214 x 1023 Pb atoms = 207.2 g
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
To determine the mass of 235 x 1024 atoms of Cu well need to consider the molar mass of copper and A...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following are the financial statements of Swifty Corporation. Swifty Corporation Comparative Balance Sheets December 31 Assets 2019 2018 Cash $37,200 $19,700 Accounts receivable 33,000 18,400...
-
a. How many hydrogen and oxygen atoms are present in 1 molecule of H2O? b. How many moles of hydrogen and oxygen atoms are present in 1 mol H2O? c. What are the masses of hydrogen and oxygen in 1.0...
-
What is the mass of 1.06 x 1024 atoms of zinc? How many atoms of mercury are present in 5.00 mL of mercury if its density is 13.55 g/mL? How many atoms of copper are present in 50.0 g of copper (Cu)?
-
In Exercises 1138, use the given conditions to write an equation for each line in point-slope form and slope-intercept form. Slope = -5, passing through (-4, -2)
-
Tondamakers produced and sold 1,000 Tonda riding lawnmowers in Year 2. Relevant data follow: Actual Results for Year 2: Direct Materials: 11,000 Pounds at $19...
-
Examples of some operational guidelines used by accountants follow. 1. The treasurer of Sweet Grapes Corp. would like to prepare financial statements only during downturns in the company's wine...
-
26. Describe assets that are considered to be listed property. Why do you think Congress requires them to be listed?
-
Mason Textile Company manufactures high-quality bed sheets and sells them in sets to a well-known retail company for $64 a set. Mason has sufficient capacity to produce 150,000 sets of sheets...
-
Which statement is most correct based on the information below? Return 2019 13.09% 2018 -5.80% 2017 8.07% 2016 10.83% The arithmetic mean is larger than the geometric mean The geometric mean is...
-
Samples of pure carbon weighing 3.62, 5.91, and 7.07 g were burned in an excess of air. The masses of carbon dioxide obtained (the sole product in each case) were 13.26, 21.66, and 25.91 g,...
-
In Example 2-1, we established that the mass ratio of magnesium to magnesium oxide is 0.455 g magnesium/ 0.755 g magnesium oxide. (a) What is the ratio of oxygen to magnesium oxide, by mass? (b) What...
-
If the farm uses its resources efficiently, what is the opportunity cost of an increase in chicken production from 300 pounds to 500 pounds a year? Explain your answer. Use the table, which shows a...
-
What are the problems (positive effects, limitations) in the development of the modern courtyard economy? (Please provide 3-5 citations) (Please use examples except China) Resource:...
-
Generate a Development Plan for the Bike-sharing Kiosks system project along with a change control Control management identify how the change will occur. For example: Who is responsible for managing...
-
With therapid development oftheinformation society, computer technology has been widely used in all walks of life. Computer applications are more and more popular in the health-care field, such as...
-
The COVID-19 pandemic has seriously disrupted skills development around the world. Mondelez International, Inc., a global snacking powerhouse also gets the big impacts about the training in the...
-
SwissAir Founded in 1931, SwissAir was the national airline of Switzerland until it was dissolved in 2002.For most of its existence, SwissAir was a very respected, stable, and profitable business,...
-
Use the recursion relation (4.46) to find the v = 3 normalized harmonic-oscillator wave function.
-
As water moves through the hydrologic cycle, water quality changes are common because of natural phenomena or anthropogenic pollution. Using Figure 11.1, describe how water-quality changes occur...
-
Intangible Amortization Presented below is selected information for Palmiero Company. 1. Palmiero purchased a patent from Vania Co. for $1,500,000 on January 1, 2008. The patent is being amortized...
-
Correct Intangible Asset Account As the recently appointed auditor for Hillary Corporation, you have been asked to examine selected accounts before the 6-month financial statements of June 30, 2010,...
-
Recording and Amortization of Intangibles Power glide Company, organized in 2009, has set up a single account for all intangible assets . The following summary discloses the debit entries that have...
-
Which one of the following is the primary source of a firm's value? A. Common stock value increases B. Operating cash flows C. Cash from investing and financing sources D. Net income
-
9 of 10. For a return filed in 2023, what is the maximum penalty the IRS can assess against a paid tax return preparer who fails to satisfy the due diligence requirements when preparing a return for...
-
Critical Thinking Problem 3 . 2 ( Algo ) Sole Proprietorship LO 3 - 1 , 3 - 2 , 3 - 3 , 3 - 4 , 3 - 5 , 3 - 6 Sara - Jayne Parsons is an architect who operates her own business. The accounts and...

Study smarter with the SolutionInn App


