Absorbance is a measure of the proportion of monochromatic (single-color) light that is absorbed as the light
Question:
Absorbance is a measure of the proportion of monochromatic (single-color) light that is absorbed as the light passes through a solution. An absorption spectrum is a graph of absorbance as a function of wavelength. High absorbances correspond to large proportions of the light entering a solution being absorbed. Low absorbances signify that large proportions of the light are transmitted. The absorption spectrum of [Ti(H2O)6]3+(aq) is shown in Figure 24-26(a).
Figure 24-26(a)
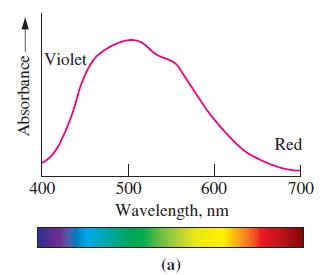 (a) Describe the color of light that [Ti(H2O)6]3+(aq) absorbs most strongly, and the color of the solution.
(a) Describe the color of light that [Ti(H2O)6]3+(aq) absorbs most strongly, and the color of the solution.
(b) Describe the electron transition responsible for the absorption peak, and determine the energy associated with this absorption.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





