According to Example 14-1, the mass percent ethanol in a particular aqueous solution is less than the
Question:
According to Example 14-1, the mass percent ethanol in a particular aqueous solution is less than the volume percent in the same solution. Explain why this is also true for all aqueous solutions of ethanol. Would it be true of all ethanol solutions, regardless of the other component? Explain.
Example 14-1
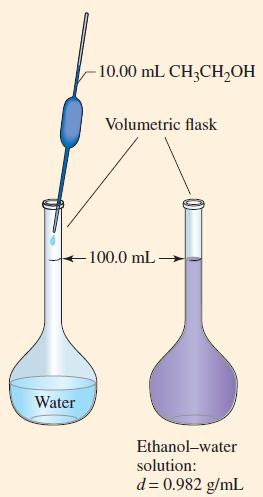
An ethanol–water solution is prepared by dissolving 10.00 mL of ethanol, CH3CH2OH (d = 0.789 g/mL), in a sufficient volume of water to produce 100.0 mL of a solution with a density of 0.982 g/mL(Fig. 14-1). What is the concentration of ethanol in this solution expressed as
(a) Volume percent;
(b) Mass percent;
(c) Mass/volume percent;
(d) Mole fraction;
(e) Mole percent;
(f) Molarity;
(g) Molality?
Figure 14-1
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





