An ethanolwater solution is prepared by dissolving 10.00 mL of ethanol, CH 3 CH 2 OH (d
Question:
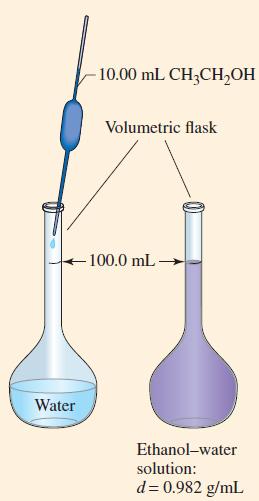
An ethanol–water solution is prepared by dissolving 10.00 mL of ethanol, CH3CH2OH (d = 0.789 g/mL), in a sufficient volume of water to produce 100.0 mL of a solution with a density of 0.982 g/mL(Fig. 14-1). What is the concentration of ethanol in this solution expressed as
(a) Volume percent;
(b) Mass percent;
(c) Mass/volume percent;
(d) Mole fraction;
(e) Mole percent;
(f) Molarity;
(g) Molality?
Figure 14-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





