An unknown white solid consists of two compounds, each containing a different cation. As suggested in the
Question:
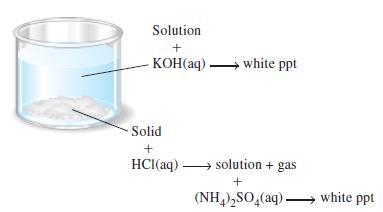
An unknown white solid consists of two compounds, each containing a different cation. As suggested in the illustration, the unknown is partially soluble in water. The solution is treated with NaOH(aq) and yields a white precipitate. The part of the original solid that is insoluble in water dissolves in HCl(aq) with the evolution of a gas. The resulting solution is then treated with (NH4)2SO4(aq) and yields a white precipitate.
(a) Is it possible that any of the cations Mg2+, Cu2+, Ba2+, Na+, or NH4+ were present in the original unknown? Explain your reasoning.
(b) What compounds could be in the unknown mixture (that is, what anions might be present)?
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





