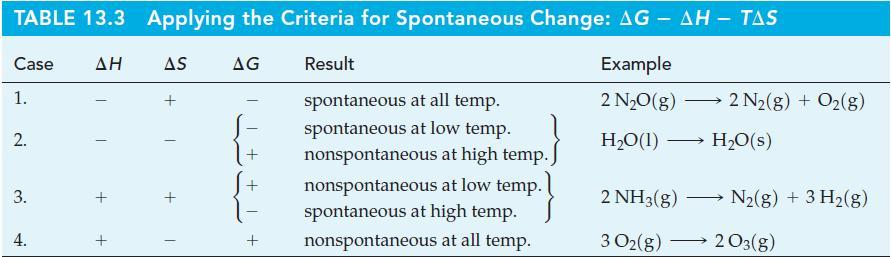
Assess the feasibility of the reaction by determining each of the following quantities for this reaction at
Question:
Assess the feasibility of the reaction
![]()
by determining each of the following quantities for this reaction at 25 °C.
(a) ΔrS° (The standard molar entropy of N2F4(g) is 301.2 J mol-1 K-1.)
(b) ΔrH° (Use data from Table 10.3 and F—O and N—F bond energies of 222 and 301 kJ mol-1, respectively.)
(c) ΔrG°
Is the reaction feasible? If so, is it favored at high or low temperatures?
Table 10.3
Transcribed Image Text:
N₂H4(g) + 2 OF2(g) N₂F4(g) + 2 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To assess the feasibility of the reaction N2H4g 2 OF2g N2F4g 2H2Og at 25C well calculate the standar...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) At what temperature will the formation of NO 2 (g) from NO(g) and O 2 (g) have Kp = 1.50 x 10 2 ? For the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g) at 25 C, r H = -114.1 kJ mol -1 , and r S =...
-
At what temperature will the equilibrium constant for the formation of NOCl(g) be K = 1.00 x 10 3 ? Data for this reaction at 25 C are 2 NO(g) + Cl(g) 2 NOCI(g) A.G = -40.9 kJ mol- AH = -77.1 kJ mol-...
-
For parts (a) and (b) of this problem, use the following standard reduction potentials, free energies, and nonequilibrium concentrations of reactants and products: ATP = 3.10 mMPi = 5. mM 5.90ADP =...
-
Suppose that Q(x, y) is a function such that 1/Q(x, y) is continuous for all (x, y). Which of the following statements are true? (a) Q(x, y) is continuous for all (x, y). (b) Q(x, y) is continuous...
-
Mussatto Corporation produces snowboards. The following per unit cost information is available: direct materials $12; direct labor $8; variable manufacturing overhead $6; fixed manufacturing overhead...
-
Two identical batteries are connected in series in a circuit with a single resistor. V = 0 V at the negative terminal of the lower battery. Rank in order, from highest to lowest, the potentials VA to...
-
What is the difference between elaborative rehearsal and maintenance rehearsal, in terms of (a) the procedures associated with each type of rehearsal and (b) their effectiveness for creating...
-
Retail Inventory Method ?Conventional and LIFO Robinson Company began operations late in 2010 and adopted the conventional retail inventory method. Because there was no beginning inventory for 2010...
-
On January 2, 2021, Petronila Company leased several machines from Petrakos Corporation under a three-year lease agreement. The lease calls for semiannual payments of $15,000 each, payable on June 30...
-
For each of the following reactions, write down the relationship between K and either K p or K c , as appropriate. (a) 2 SO(g) + O(g) = - 2 SO3(g) 1/11 (b) HI(g) = (c) NH4HCO3(s) I2(g) NH3(g) + CO(g)...
-
Write an equation for the combustion of one mole of benzene, C 6 H 6 (l), and determine at 298 K if the products of the combustion are (a) CO 2 (g) and H 2 O(l), and (b) CO 2 (g) and H 2 O(g)....
-
Find the equation of each of the circles from the given information. The origin and (6, 8) are the ends of a diameter
-
The pay disparity is due to several reasons, one of the main ones being the old stereotypes based on the archetype of the man as the breadwinner of the family. Women are usually hired at a lower...
-
Prepare Balance Sheet: To do this activity you are required to assume the amount and line items that are to be shown on the balance sheet of your business selling homemade articles. Using the...
-
You have a "Consent to Use E-mail Communication" on file for this patient. Draft a short e-mail to her about her lab and chest X-ray results, requesting she contact the office by phone or e-mail to...
-
+ Given f(x) = x - 9 and g(x) = x+9, complete the following. (a) Find f(g(x)) and g(f(x)). (Simplify your answers completely.) f(g(x)) = g(f(x)) = (b) What does this tell us about the relationship...
-
Case Study - Rhonda Rhonda is a 28-year-old woman who has been referred to your agency by a local probation officer. Rhonda reported that she has "fired" three counselors in the past and most...
-
For the balanced circuit in Fig. 12.55, Vab = 125 0° V. Find the line currents IaA, IbB, and IcC. 0A 24 Three-phase -connected generator -j15 IDB 24 (+)phase sequence -j15 24
-
Write a while loop that uses an explicit iterator to accomplish the same thing as Exercise 7.3. Exercise 7.3. Write a for-each loop that calls the addInterest method on each BankAccount object in a...
-
Account analysis method. Gower, Inc., a manufacturer of plastic products, reports the following manufacturing costs and account analysis classification for the year ended December 31, 2009. Gower,...
-
Estimating a cost function, high-low method. Reisen Travel offers helicopter service from suburban towns to John F. Kennedy International Airport in New York City. Each of its 10 helicopters makes...
-
Estimating a cost function, high-low method. Laurie Daley is examining customer-service costs in the southern region of Capitol Products. Capitol Products has more than 200 separate electrical...
-
A taxpayer can claim a personal exemption for any person he or she provides over 50 percent of support during the tax year. True False
-
Holding other variables constant, a recession in the US economy should: Select one: A) cause a depreciation of the dollar because the nominal interest rate in the United States would increase b)...
-
CROSS RATES The table below gives the exchange rates between U.S. dollar, British pound and Swedish krona. What is the exchange rate between Swedish kronas and pounds? Round your answer to two...

Study smarter with the SolutionInn App


