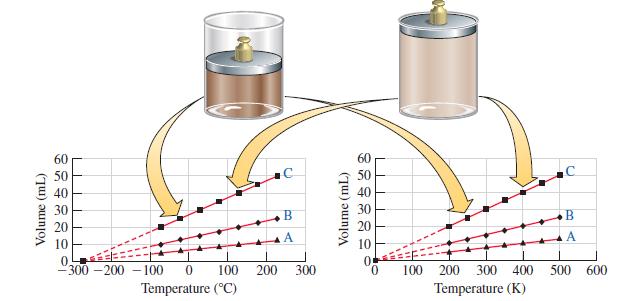
Assume the following initial conditions for the graphs labeled A, B, and C in Figure 6-7. (A)
Question:
Assume the following initial conditions for the graphs labeled A, B, and C in Figure 6-7.
(A) 10.0 mL at 400 K;
(B) 20.0 mL at 400 K;
(C) 40.0 mL at 400 K.
Use Charles’s law to calculate the volume of each gas at 0, -100, -200, -250, and -270 °C. Show that the volume of each gas becomes zero at -273.15 °C.
Figure 6-7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





