(a) Use the value of the van der Waals constant b for CH 4 (g), given in...
Question:
(a) Use the value of the van der Waals constant b for CH4(g), given in Table 6.5, to estimate the radius of the CH4 molecule. (See Exercise 89.) How does your estimate of the radius compare with the value r = 228 pm, obtained experimentally from an analysis of the structure of solid methane?
(b) The density of CH4(g) is 66.02 g mL-1 at 100 bar and 325 K. What is the value of the compressibility factor at this temperature and pressure?
Exercise 89
Use the value of the van der Waals constant b for He(g) given in Table 6.5, to estimate the radius, r, of a single helium atom. Give your answer in picometers.
Table 6.5
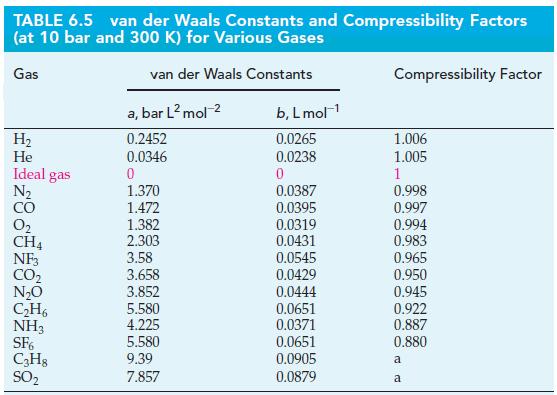
TABLE 6.5 van der Waals Constants and Compressibility Factors (at 10 bar and 300 K) for Various Gases van der Waals Constants Gas H₂ He Ideal gas N₂ CO 0₂ CH4 NF3 CO₂ N₂O C₂H6 NH3 SF6 C3H8 SO₂ a, bar L² mol-² 0.2452 0.0346 0 1.370 1.472 1.382 2.303 3.58 3.658 3.852 5.580 4.225 5.580 9.39 7.857 b, L mol-¹ 0.0265 0.0238 0 0.0387 0.0395 0.0319 0.0431 0.0545 0.0429 0.0444 0.0651 0.0371 0.0651 0.0905 0.0879 Compressibility Factor 1.006 1.005 1 0.998 0.997 0.994 0.983 0.965 0.950 0.945 0.922 0.887 0.880 a a
Step by Step Answer:
The van der Waals equation for a real gas is given by P Vb RT where P is the pressure V is the molar ...View the full answer



General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Students also viewed these Sciences questions
-
Use the value of the van der Waals constant b for He(g) given in Table 6.5, to estimate the radius, r, of a single helium atom. Give your answer in picometers. Table 6.5 TABLE 6.5 van der Waals...
-
A certain gas obeys the van der Waals equation with a =0.76 m6 Pa mol-2, its volume is found to be 4.00 X 10-4 m3 mol-1 at 288 K and 4.0 MPa. From this information calculate the van der Waals...
-
The volume of a spherical molecule can be estimated as V = b/(4N A ), where b is the van der Waals parameter for the excluded molar volume and N A is Avogadros number. Justify this relationship by...
-
If you uncover critically important information (the sort that could make or break your company) that is from a credible source and appears to be unbiased, well documented, current, and complete but...
-
In addition to your regular responsibilities, your supervisor has just assigned you to be in charge of your organizations annual golf tournament. It is expected that 100 to 150 employees will enter...
-
Daniel Hagell operates a gardening consulting business. His business had the following selected transactions: a. Paid cash for miscellaneous items, $150. b. Paid an insurance broker $900 in insurance...
-
Know what skills are essential for eff ective leadership
-
Diem Corporation manufactures fertilizer products that are sold through a network of external sales agents. The agents are paid a commission of 20% of revenues. Diem is considering replacing the...
-
In March, Blossom Company completes Jobs 10 and 11. Job 10 cost $24,800 and Job 11 $37,200. On March 31, Job 10 is sold to the customer for $43,400 in cash. Journalize the entries for the completion...
-
Assume the following initial conditions for the graphs labeled A, B, and C in Figure 6-7. (A) 10.0 mL at 400 K; (B) 20.0 mL at 400 K; (C) 40.0 mL at 400 K. Use Charless law to calculate the volume of...
-
Refer to Example 6-17. Recalculate the pressure of Cl 2 (g) by using both the ideal gas equation and the van der Waals equation at the temperatures (a) 100 C; (b) 200 C; (c) 400 C. From the results,...
-
A molten 50.0-kg quantity of gold at 1063 C is to be solidified without changing its temperature. What must be done?
-
Vine plc. produces a single product. The following information on inventory, purchases, and sales are available for the month of January 2018. DATE TRANSACTION NUMBER OF UNITS UNIT COST...
-
4. Thinking Ahead (2 points): Project 1 involves the analysis of a bicycle pedal. Consider the bicycle shown below. If a rider places their full weight on the pedal when it is in the horizontal...
-
On January 1, 2023, Martineau Corp. issued a 5-year, 5% installment note payable for $118,000 to finance upgrading its current equipment. The company's year end is December 31. The repayment of...
-
Multiply. 2 x-x-2 3x-3 2 x+2x-3 x+1 Simplify your answer as much as possible.
-
Explain the processes of querying a relational database and define Big Data and explain its basic characteristics. Compare and contrast the major types of networks. - Identify the fundamentals of...
-
Explain why it would be incorrect to calculate the experimental ground-state energy of lithium by taking - (E2s + 2E1s), where E2s is the experimental energy needed to remove the 2s electron from...
-
Study the pictures/images below. Obviously these was focus on LT sociology, anthropology and poltical science. Try to do some analysis by finding clues that are synonymous with the main concepts....
-
Explain how a non-consolidated subsidiary can be a form of off-balance-sheet financing.
-
Where can authoritative i GAAP guidance related to liabilities are found?
-
Briefly describe some of the similarities and differences between U.S. GAAP and iGAAP with respect to the accounting for liabilities.
-
Adirondack Marketing Inc. manufactures two products, A and & Presently, the company uses a single plantwide factory overhead rate for allocating overhead to products, However, management is...
-
9. value: 10.00 points < Question 9 (of 10) P3-18 Using the Du Pont Identity [LO3] Y3K, Inc., has sales of $4,500, total assets of $3,460, and a debt-equity ratio of 1.40. If its return on equity is...
-
Consider the simple income statement model below. The company would like donate 15% of its net income to charity. How much will they contribute? Contribution Rate 15% Income Tax Rate 40% Circular...

Study smarter with the SolutionInn App


