At 65 C, the half-life for the first-order decomposition of N 2 O 5 (g) is 2.38
Question:
At 65 °C, the half-life for the first-order decomposition of N2O5(g) is 2.38 min.
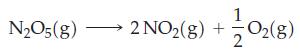
If 1.00 g of N2O5 is introduced into an evacuated 15 L flask at 65 °C,
(a) What is the initial partial pressure, in mmHg, of N2O5(g)?
(b) What is the partial pressure, in mmHg, of N2O5(g) after 2.38 min?
(c) What is the total gas pressure, in mmHg, after 2.38 min?
Transcribed Image Text:
N₂O5 (g) 2 NO2(g) + O₂(g) 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a To calculate the initial partial pressure of N2O5g we can use the ideal gas law PV nRT where P is ...View the full answer

Answered By

Simon kingori
I am a tier-one market researcher and content developer who has been in this field for the last six years. I’ve run the freelancing gamut; from market research, data mining and SEO/SMM to copywriting, Content Development, you name it, I’ve done it. I’m extremely motivated, organized and disciplined – you have to be to work from home. My experience in Freelancing is invaluable- but what makes me a cut above the rest is my passion to deliver quality results to all my clients- it’s important to note, I've never had a dissatisfied client. Backed by a Masters degree in Computer Science from MOI university, I have the required skill set and burning passion and desire to deliver the best results for my clients. This is the reason why I am a cut above the rest. Having taken a Bsc. in computer science and statistics, I deal with all round fields in the IT category. It is a field i enjoy working in as it is dynamic and new things present themselves every day for research and exploration.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
A sample of gaseous PCl5 was introduced into an evacuated flask so that the pressure of pure PCl5 would be 0.50 atm at 523 K. However, PCl5 decomposes to gaseous PCl3 and Cl2, and the actual pressure...
-
If 10.00 g of water are introduced into an evacuated flask of volume 2.500 L at 65C, calculate the mass of water vaporized. (Assume that the volume of the remaining liquid water is negligible; the...
-
(1) Choose all of the following statements that are correct about the time evolution of a general wave function: (I) The time evolution of a general wave function is governed by the Hamiltonian...
-
Largest Company acquired Large Company on January 1. As part of the acquisition, $10,000 in goodwill was recognized; this goodwill was assigned to Largest's Production reporting unit. During the...
-
A mercury lamp contains 0.0055 g of Hg vapor in a volume of 15.0 mL. If the operating temperature is 2,800 K, what is the pressure of the mercury vapor?
-
Define activities as the basis for developing project schedules? LO.1
-
You are a manager at Glass Inc.a mirror and window supplier. Recently, you conducted a study of the production process for your single- side encapsulated window. The results from the study are...
-
When Job 117 was completed, direct materials totaled $32,830; direct labor, $38,799; and factory overhead, $27,855. A total of 2,926 units were produced at a per-unit cost of a.$99,484 b.$71,629...
-
An alternative mechanism of the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g) follows. Show that this mechanism is consistent with the rate law, equation (20.24). Eq. 20.24 Fast: Slow: Overall: NO(g) + O(g)...
-
(A) In a proposed two-step mechanism for the reaction CO(g) + NO 2 (g) CO 2 (g) + NO(g), the second, fast step is NO 3 (g) + CO(g) NO 2 (g) + CO 2 (g). What must be the slow step? What would you...
-
Measurements were made for a sample of adult men. Assume that the association between their hand length and foot length is linear. Output for predicting foot length from hand length is provided from...
-
Management is what tradition used to call a liberal art: "liberal" because it deals with the fundamentals of knowledge, self-knowledge, wisdom, and leadership; "art" because it is a practice and...
-
Draft a five hundred and twenty five- to seven hundred-word internal communication planthat appropriately details your proposed solution to the internal team at CVS PHARMACY. In your communication...
-
Christopher Awnings was founded by Christopher Aminim in the early days of the retirement boom in the Okanagan to build and install custom retractable awnings for retirees to keep the sun out of the...
-
Leaders are responsible for making decisions that have long-term ramifications; thus, making the appropriate decisions can be stressful and leaders' decisions may vary. They often enhance employee...
-
Employee longevity A large insurance company has developed a model to identify the factors associated with employee turnover. The dependent variable is number of years an employee stays with the...
-
Explain why negative springback does not occur in air bending of sheet metals.
-
Provide an example of an aggressive accounting practice. Why is this practice aggressive?
-
Glendo Industries' balance sheet at December 31, 2010, is presented below and on the next page. Additional information accumulated for the budgeting process is as follows.Budgeted data for the year...
-
Suppan Farm Supply Company manufactures and sells a fertilizer called Basic II. The following data are available for preparing budgets for Basic II for the first 2 quarters of 2010. 1. Sales: Quarter...
-
Durham Inc. is preparing its annual budgets for the year ending December 31, 2010. Accounting assistants furnish the following data. An accounting assistant has prepared the detailed manufacturing...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


