An alternative mechanism of the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g)
Question:
An alternative mechanism of the reaction 2 NO(g) + O2(g) → 2 NO2(g) follows. Show that this mechanism is consistent with the rate law, equation (20.24).
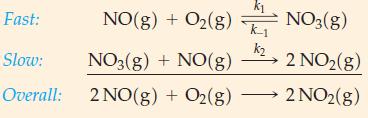
Eq. 20.24
![]()
Transcribed Image Text:
Fast: Slow: Overall: NO(g) + O₂(g) 0₂(g) NO3(g) + NO(g) 2 NO(g) + O₂(g) K-1 k₂ NO3(g) 2 NO₂(g) 2 NO2(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Analyze In this type of problem we begin by identifying the slow step which is typically given and u...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Suppose that the reaction in Example 20-8 is first order with a rate constant of 0.12 min -1 . Starting with [A] 0 = 1.00 M, will the curve for [A] versus t for the first-order reaction cross the...
-
The reaction 2NO(g) + O2(g) 2NO2(g) exhibits the rate law Rate = k[NO]2[O2] Which of the following mechanisms is consistent with this rate law? a. NO + O2 NO2 + O Slow O + NO NO2 Fast b. NO + O2 ...
-
In this case we are concerned with an employers genderbased fetal-protection policy. May an employer exclude a fertile female employee from certain jobs because of its concern for the health of the...
-
On December 31, 2021, there is a batch of commodities sold under FOB destination conditions in the shipping area, and this batch of commodities is not included in the inventory count middle. There is...
-
On December 31, 2011, Debenham Corporation sold for $15,000 an old machine having an original cost of $84,000 and a book value of $9,000. The terms of the sale were as follows: $3,000 down payment,...
-
A gas has an initial volume of 685 mL and an initial temperature of 29C. What is its new temperature if volume is changed to 1.006 L? Assume pressure and amount are held constant.
-
Discuss the process of planning schedule management? LO.1
-
Transactions related to revenue and cash receipts completed by Main Line Inc. during the month of August 2012 are as follows: Aug. 2. Issued Invoice No. 512 to Boston Co., $780. 4. Received cash from...
-
PROBLEM 1 A . For the properties listed below compute the depr e ciation taken over the useful life of the property. Assume that all property is post-1986 property. B . Include columns for the...
-
The initial rate of the reaction A + B C + D is determined for different initial conditions, with the results listed in the table. (a) What is the order of reaction with respect to Aand to B? (b)...
-
At 65 C, the half-life for the first-order decomposition of N 2 O 5 (g) is 2.38 min. If 1.00 g of N 2 O 5 is introduced into an evacuated 15 L flask at 65 C, (a) What is the initial partial pressure,...
-
In Exercises, find the absolute extrema if they exist, as well as all values of x where they occur. f(x) = X x + 1
-
PART 1: DIGITAL TECHNOLOGY: Describe the key digital technology groups studied in this course and include a discussion of two examples for each group. PART 2: SOCIAL MEDIA: As studied in this course,...
-
Doing a strategic analysis of GraceKennedy Limited, What is the current level of its economic performance, an indication of the factors responsible for the current performance and recommendations for...
-
Dynamic capability is the ability for change and manage corporate learning. It allows an enterprise to adapt, develop and respond to future opportunities and discontinuous technologies. Innovation...
-
What potential solutions can organizations try to help support the adoption of a CDSS? In other words, what are some ways an organization can address the factors of implementation obstruction that...
-
Identify and briefly describe and discuss the three most important factors in building and maintaining trust among virtual global team members. Include in your discussion how you can leverage these...
-
Give examples of products in which the presence of beads is beneficial or even necessary.
-
Explain the regulation of the secretions of the small intestine.
-
Colt Industries had sales in 2010 of $6,400,000 and gross profit of $1,100,000. Management is considering two alternative budget plans to increase its gross profit in 2011. Plan A would increase the...
-
Haas Company prepares monthly cash budgets. Relevant data from operating budgets for 2011 are: All sales are on account. Collections are expected to be 50% in the month of sale, 30% in the first...
-
The budget committee of Deleon Company collects the following data for its San Miguel Store in preparing budgeted income statements for May and June 2011. 1. Sales for May are expected to be...
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


