Suppose that the reaction in Example 20-8 is first order with a rate constant of 0.12 min
Question:
Suppose that the reaction in Example 20-8 is first order with a rate constant of 0.12 min-1. Starting with [A]0 = 1.00 M, will the curve for [A] versus t for the first-order reaction cross the curve for the second-order reaction at some time after t = 0? Will the two curves cross if [A]0 = 2.00 M? In each case, if the curves are found to cross, at what time will this happen?
Example 20-8
An alternative mechanism of the reaction 2 NO(g) + O2(g) → 2 NO2(g) follows. Show that this mechanism is consistent with the rate law, equation (20.24).
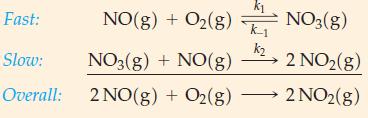
Eq. 20.24
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





