Determine the molar solubility of lead(II) azide, Pb(N 3 ) 2 , in a buffer solution with
Question:
Determine the molar solubility of lead(II) azide, Pb(N3)2, in a buffer solution with pH = 3.00, given that
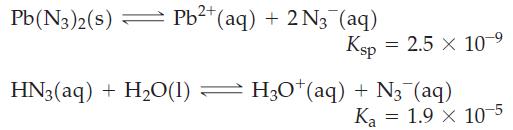
Transcribed Image Text:
Pb(N3)2(s) Pb²+ (aq) + 2N3 (aq) = Ksp 2.5 x 10 ⁹ HN3(aq) + H₂O(1) — H3O*(aq) + N₂ (aq) = Ka 1.9 x 10-5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
To determine the molar solubility of leadII azide PbN32 in a buffer solution with pH 300 we need ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A saturated solution of lead iodate in pure water has an iodate-ion concentration of 8.0 10-5 M. a. What is the molar solubility of lead iodate in a 0.15 M lead nitrate solution at the same...
-
The solubility of CaCO 3 is pH dependent. a) Calculate the molar solubility of CaCO 3 (K sp = 4.5 10 -9 ) neglecting the acidbase character of the carbonate ion. (b) Use the expression for the CO 3...
-
The K sp of Ca 3 (PO 4 ) 2 is 2.0 10 33 . Determine its molar solubility in 0.05 M (NH 4 ) 3 PO 4 . Compare your answer to the molar solubility of Ca3(PO4)2 in water, which we calculated in Example...
-
Using the framework of the marketing mix, appraise the marketing tactics of Boo.com in the areas of Product, Pricing, Place, Promotion, Process, People and Physical Evidence.
-
Transistor Electronics makes all of its sales on credit and accounts for them using the installment sales method. For simplicity, assume that all sales occur on the first day of the year and that all...
-
Solve each of the following problems by direct integration: a. 4x = 3t x(0) = 2 b. 5x = 2e4t x(0) = 3 c. 3* = 5t x(0) = 2 * (0) = 7 d. 4x = 7e %3D x(0) = 4 * (0) = 2 e. * = 0 x(0) = 2 * (0) = 5
-
Eye anatomy of giraffes. Giraffes are believed to have excellent vision. The journal African Zoology (Oct. 2013) published a study of giraffe eye characteristics. Data were collected for a sample of...
-
Various types of accounting changes can affect the auditor's report. a. Briefly describe the rationale for having accounting changes affect the auditor's report and the auditor's responsibility in...
-
Wildcat, Incorporated, has estimated sales ( in millions ) for the next four quarters as follows: Sales for the first quarter of the following year are projected at $ 1 4 5 million. Accounts...
-
Assume that the seawater sample described in Example 18-6 contains approximately 440 g Ca 2+ per metric ton (1 metricton = 10 3 kg; density of seawater = 1.03 g/mL) (a) Should Ca(OH) 2 (s)...
-
Calculate the molar solubility of Mg(OH) 2 in 1.00 M NH 4 Cl(aq).
-
What is moral leadership?
-
Vaporization of mixtures of hexane and octane. Using the T-x-y diagram (Figure 1) on the next page, determine the temperature, amounts, and compositions of the vapor and liquid phases at 1 atm for...
-
what should p&g do to replace lafley when he retires a second time? what actions should they take to prepare for the succession?
-
What do these terms mean? What would be the currencies (one at a time) from two total UN Member States (other than the EURO, USD, JPY, GBP, or CHF). What would be the foreign currencies and how they...
-
How do social identity processes, such as categorization, identification, and comparison, influence team cohesion and performance within complex organizational environments ?
-
How do calculate sales forecast and expense forecast for several years
-
Following Quiz 1.3, use MATLAB, but not the randi function, to generate a vector T of 200 independent test scores such that all scores between 51 and 100 are equally likely.
-
Time Travel Publishing was recently organized. The company issued common stock to an attorney who provided legal services worth $25,000 to help organize the corporation. Time Travel also issued...
-
Tony Masasi started his own consulting firm, Masasi Company, on June 1, 2010. The trial balance at June 30 is shown below. In addition to those accounts listed on the trial balance, the chart of...
-
Neosho River Resort opened for business on June 1 with eight air-conditioned units. Its trial balance before adjustment on August 31 is as follows. In addition to those accounts listed on the trial...
-
Fernetti Advertising Agency was founded by John Fernetti in January of 2009. Presented on page 134 are both the adjusted and unadjusted trial balances as of December 31, 2010. Instructions(a)...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


