Estimate the percent dissociation of Cl 2 (g) into Cl(g) at 1 atm total pressure and 1000
Question:
Estimate the percent dissociation of Cl2(g) into Cl(g) at 1 atm total pressure and 1000 K. Use data from Appendix D and equations found elsewhere in this text, as necessary.
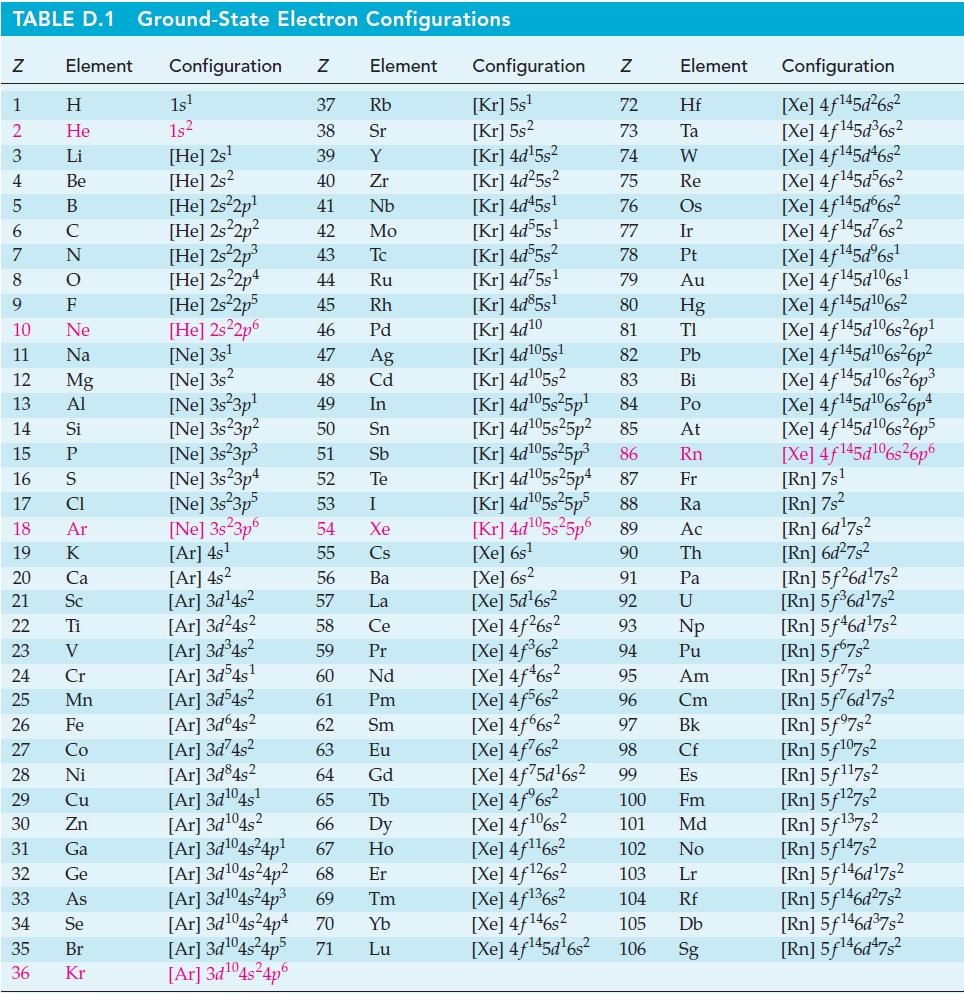
Transcribed Image Text:
TABLE D.1 Ground-State Electron Configurations Element Configuration Z Z 1 2 3 4 5 6 7 8 9 HIG&LUZONSUZ SE> 0 ≤ 2 8 2 3 5 3 3 2 2 5 2 He 10 11 12 13 14 15 16 17 18 19 20 21 Sc 22 23 24 25 Mn Mg 26 27 28 Ni 29 30 31 32 33 34 35 36 Zn Ga Ge 1s¹ 1s² [He] 2s¹ [He] 2s2 [He] 2s²2p¹ [He] 2s²2p² [He] 2s²2p³ [He] 2s22p4 [He] 2s²2p5 [He] 2s²2p6 [Ne] 3s¹ [Ne] 3s2 [Ne] 3s 3p¹ [Ne] 3s 3p² 37 Rb 38 Sr 39 Y 40 Zr 41 Nb 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 Sb 52 Te 53 54 55 56 57 58 59 60 61 62 [Ar]3d²4s² 63 [Ar] 3d845² 64 66 [Ar]3d¹04s¹ 65 [Ar]3d¹04s2 [Ar]3d¹04s²4p¹ [Ar] 3d¹04s²4p² 68 67 [Ar] 3d¹04s²4p³ [Ne] 3s²3p³ [Ne] 3s23p4 [Ne] 3s²3p5 [Ne] 3s 3p6 [Ar] 4s¹ [Ar] 4s² [Ar]3d¹4s² [Ar]3d²4s² [Ar] 3d³4s² [Ar]3d54s¹ [Ar]3d³4s² [Ar] 3d64s² Element [Ar]3d¹04s²4p4 70 [Ar]3d¹04s²4p5 71 [Ar]3d¹04s²4p6 I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er 69 Tm Yb Lu Configuration Z [Kr] 5s¹ [Kr] 5s² [Kr] 4d¹5s² [Kr] 4d²5s² [Kr] 4d45s¹ [kr] 4d55s¹ [Kr] 4d55s² [Kr] 4d75s¹ [Kr] 4d85s1 [Kr] 4d10 [Kr] 4d105s1 [Kr] 4d¹05s² [Kr] 4d¹05s²5p¹ [Kr] 4d¹05s25p² [kr] 4d¹05s²5p³ [kr] 4d¹05s25p4 [Xe] 6s² [Xe] 5d¹6s² [Xe] 4f²6s² [Xe] 4f³6s² [Xe] 4f46s2 [Xe] 4f6s2 [Xe] 4f6s2 [Xe] 4f²6s² [Xe] 4f75d¹6s² [Xe] 4f%s2 [Xe] 4f106s2 [Xe] 4f¹¹6s² NRNKERKR [Xe] 4f126s2 [Xe] 4f136s2 [Xe] 4f146s2 [Xe] 4f¹45d¹6s² 72 Hf 73 Ta W 74 75 Re 76 Os 77 Element 78 79 80 81 82 83 84 85 86 87 Fr [Kr] 4d¹05s²5p5 88 Ra [Kr] 4d¹05s²5p6 89 Ac [Xe] 6s¹ 90 Th 91 92 93 94 95 96 97 98 99 Ir Pt Au Hg TI Pb Bi Po At Rn Pa U Np Pu Am Cm Bk Cf Es 100 Fm 101 Md 102 No 103 Lr 104 Rf 105 Db 106 Sg Configuration [Xe] 4f¹45d²6s² [Xe] 4f145d³6s² [Xe] 4f145d46s2 [Xe] 4f145d56s2 [Xe] 4f145d6s2 [Xe] 4f¹45d²6s² [Xe] 4f¹45dº6s¹ [Xe] 4f145d106s1 [Xe] 4f145d106s2 [Xe] 4f145d6s26p* [Xe] 4f145d106s36p? [Xe] 4f145d16s?6p3 [Xe] 4f145d6s®6p* [Xe] 4f145d16s26p5 [Xe] 4f145d106s26p6 [Rn] 7s¹ [Rn] 7s² [Rn] 6d¹7s² [Rn] 6d²7s² [Rn] 5f26d¹7s² [Rn] 5f³6d¹7s² [Rn] 5f46d¹7s2 [Rn] 5f67s² [Rn] 5f77s² [Rn] 5f76d¹7s² [Rn] 5f97s2 [Rn] 5f107,2 [Rn] 5f117s2 [Rn] 5f¹27s² [Rn] 5f137,2 [Rn] 5f147s2 Rn] 5f¹46d¹7s² [Rn] 5f¹46d²7s² [Rn] 5f¹46d³7s2 [Rn] 5f¹46d¹7s²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from Appendix C, Figure 7.9, and Figure 7.11 to calculate the lattice energy of RbCl. Is this value greater than or less than the lattice energy of NaCl? Explain. 2372 2081 1312 1681 9 1402...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Use data from Appendix D and other information from this chapter to estimate the temperature at which the dissociation of I 2 (g) becomes appreciable [for example, with the I 2 (g) 50% dissociated...
-
Marcus is the HR manager for United Airlines, an Illinois-based company. One of his employees has recently become disabled and is unable to fulfill the essential functions of his current position,...
-
The following financial statements are available for Sherwood Real Estate Company: Sherwood Company is using these financial statements to entice investors to buy stock in the company. However, a...
-
Pam Corporation acquired all the outstanding stock of Sun Corporation on April 1, 2016, for $15,000,000, when Sun's stockholders' equity consisted of $5,000,000 capital stock and $2,000,000 retained...
-
The expected value of a discrete random variable is equal to the standard deviation of the random variable. Graphical Analysis In Exercises 912, decide whether the graph represents a discrete random...
-
The Acute Company manufactures a single product. On December 31, 2006 Acute adopted the dollar-value LIFO inventory method. It computes the inventory on that date using the dollar-value LIFO...
-
B2B Co. is considering the purchase of equipment that would allow the company to add a new product to its line. The equipment is expected to cost $379,200 with a 10-year life and no salvage value. It...
-
Despite the fact that it has the higher molecular mass, XeO 4 exists as a gas at 298 K, whereas XeO 3 is a solid. Give a plausible explanation for this observation.
-
One reaction for the production of adipic acid, HOOC(CH 2 ) 4 COOH, used in the manufacture of nylon, involves the oxidation of cyclohexanone, C 6 H 10 O, in a nitric acid solution. Assume that...
-
Why is maintaining your hospitality facility so important? LO.1
-
Based on contract law principles, do you think the jury\'s verdict against the Loewen Group for $ 5 0 0 million was appropriate? Why or why not? What factors should the jury have considered in...
-
5.) Consider you have two systems - one filled with (1kg) water and the other with (1kg) of air. Both systems are at 1000 kPa and 30 C. Determine numerically which fluid system has the larger...
-
Question 3: The partnership of Blossom, Blue, and Kingbird engaged you to adjust its accounting records and convert them uniformly to the accrual basis in anticipation of admitting Kerns as a new...
-
Instructions : Build an Excel spreadsheet using the accounting equation (Assets = Liabilities + Shareholders' Equity). Remember that each transaction has an equal effect on both the left-hand side...
-
7.3 Fill in the spreadsheet below to calculate the port- folio return and risk between Zenon and Dynamics, given the 10 years of annual returns for each stock and portfolio weights of 50/50. (a) How...
-
Find a minimal-sum-of-products representation for (a) f(w, x, y, z) = m(1, 3, 5, 7, 9) + d(10, 11, 12, 13, 14, 15) (b) f(w, x, y, z) = m(0, 5, 6, 8, 13, 14) + d(4, 9, 11) (c) f(v, w, x, y, z) = ...
-
Make an argument that Williams had a right to delay the closing until after August 1.
-
Listed here are some items found in the financial statements of Ellyn Toth, Inc. Indicate in which financial statement(s) each item would appear. (a) Service revenue. (b) Equipment. (c) Advertising...
-
A companys net income appears directly on the income statement and the retained earnings statement, and it is included indirectly in the companys balance sheet. Do you agree? Explain.
-
What is the basic accounting equation?
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


