Estimate the standard enthalpies of formation at 25 C and 1 bar of (a) OH(g); (b) N
Question:
Estimate the standard enthalpies of formation at 25 °C and 1 bar of
(a) OH(g);
(b) N2H4(g).
Write Lewis structures and use data from Table 10.3, as necessary.
Table 10.3
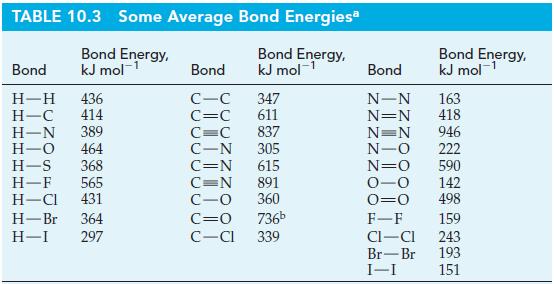
Transcribed Image Text:
TABLE 10.3 Some Average Bond Energiesa Bond Energy, kJ mol-¹ Bond Energy, kJ mol-¹ Bond H-H 436 H-C 414 H-N 389 H-O 464 H-S 368 H-F 565 H-Cl 431 H-Br 364 H-I 297 Bond C-C C=C C=C 837 C-N 305 C=N 615 347 611 C=N C-O C=O 736b C-Cl 339 891 360 Bond Energy, kJ mol-¹ 1 Bond N-N 163 N=N 418 N=N 946 -0 222 N=O 590 -O 142 0=0 498 F-F CI-CI Br-Br I-I 159 243 193 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
12H2g 12028 AH AH AH rxn OHs AH bonds brokenAH b...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpies of formation of S(g), F(g), SF4(g), and SF6(g) are 1278.8 kJ/ mol, 179.0 kJ/ mol, 775 kJ/ mol, and 1209 kJ/ mol, respectively. a. Use these data to estimate the energy of an...
-
The standard free energies of formation and the standard enthalpies of formation at 298 K for difluoroacetylene (C2F2) and hexafluorobenzene (C6F6) are For the following reaction: C6F6(g) 3C2F2(g) a....
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
In each of the homeowners forms, OA) the property coverage is the same OB) the liability coverage varies OC) both the property and liability coverage are the same OD) the property coverage varies
-
Discuss and explain whether there is valid consideration for each of the following promises: (a) A and B entered into a contract for the purchase and sale of goods. A subsequently promised to pay a...
-
Is it easier to measure the stand-alone, within-firm, or beta risk for projects such as a new delivery truck or a Home Depot warehouse?
-
What does it mean to assert that the theta of an option position is -100 per day? If a trader feels that neither a stock price nor its implied volatility will change, what type of option position is...
-
Presented here is a partial list of accounts for the total governmental funds of the City of Bukowy. What worksheet adjustments would be required to convert this information to information that the...
-
For this assignment, assume that you were discussing what you learned in this module with a friend, and they were so impressed with your knowledge that they asked you to help them evaluate their...
-
Now that you have read the chapter on estate planning, what do you recommend to Juliana and Fernando on the subject of retirement and estate planning regarding? 1. How much in Social Security...
-
Two of the following have the same shape. Which two, and what is their shape? What are the shapes of the other two? NI 3 , HCN, SO 3 2- , NO 3 - .
-
One of the chemical reactions that occurs in the formation of photochemical smog is O 3 + NO NO 2 + O 2 . Estimate r H for this reaction by using appropriate Lewis structures and data from Table...
-
Frobisher Inc. (Frobisher) uses the lower of cost and NRV rule to value its inventory. Frobishers inventory on February 28, 2017 had a cost of $1,125,000 and a NRV of $1,035,000. Required: a. By how...
-
C. Prove the following (you can use any formal induction/other theoretical method, "A" means power here): i. ii. iii. What is the time complexity recurrence relation for Fibonacci numbers? Explain it...
-
You and your partner run a small business together, with separate work roles. You are responsible for the business budget and have researched an improved budget process which you felt needs to be...
-
The actual selling expenses incurred in March 2022 by Carla Vista Company are as follows: Variable Expenses Fixed Expenses Sales commissions Advertising $14,576 Sales salaries $34,700 12.174...
-
Winston Electronics reported the following information at its annual meetings. The company had cash and marketable securities worth $1,235,740, accounts payables worth $4,160,391, inventory of...
-
Hooray Company has been manufacturing 12,000 units of Part A which is used to manufacture one of its products. At this level of production, the cost per unit is as follows: Direct materials P 4.80...
-
Write the structure for all possible dehydration products of In each case, which product do you expect to predominate? b. a.
-
Reichenbach Co., organized in 2018, has set up a single account for all intangible assets. The following summary discloses the debit entries that have been recorded during 2018 and 2019. Instructions...
-
Write down the formulas for an investments NPV and rate of return. Prove that NPV is positive only if the rate of return exceeds the opportunity cost of capital.
-
What is the net present value of a firms investment in a U.S. Treasury security yielding 5 percent and maturing in one year?
-
A parcel of land costs $500,000. For an additional $800,000 you can build a motel on the property. The land and motel should be worth $1,500,000 next year. Suppose that common stocks with the same...
-
General corporation decide to acquire or merge with another corporation, suggest a potential target company/corporation(any existing company with stocks). Justify why and explain if it is horizontal,...
-
The yield to maturity is not the compound annual rate of return earned on a debt security purchased on a given day and held to maturity. true or false
-
If 1500 is deposited at the end of each quarter in an account that earns 4% compounded quarterly, after how many quarters will the account contain 60,000? (round your answer up to the nearest quarter)

Study smarter with the SolutionInn App


