One of the chemical reactions that occurs in the formation of photochemical smog is O 3 +
Question:
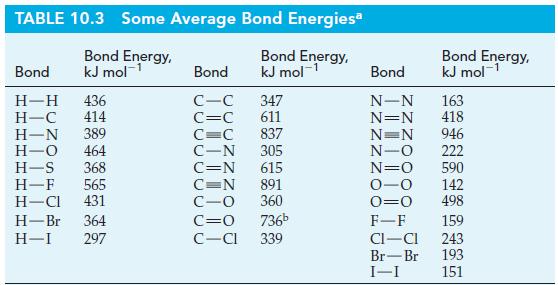
One of the chemical reactions that occurs in the formation of photochemical smog is O3 + NO → NO2 + O2. Estimate ΔrH for this reaction by using appropriate Lewis structures and data from Table 10.3.
Table 10.3
Transcribed Image Text:
TABLE 10.3 Some Average Bond Energiesa Bond Energy, kJ mol-¹ Bond Energy, kJ mol-¹ Bond H-H 436 H-C 414 H-N 389 H-O 464 H-S 368 H-F 565 H-Cl 431 H-Br 364 H-I 297 Bond C-C C=C C=C C-N 305 C=N 615 347 611 837 C=N 891 C-O 360 C=O C-Cl 736b 339 Bond Energy, kJ mol-¹ Bond N-N 163 N=N 418 N=N 946 N-O 222 N=O 0-0 0=0 F-F CI-CI Br-Br I-I 590 142 498 159 243 193 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
O3g NOg AH AH AH NO2g ...View the full answer

Answered By

Niala Orodi
I am a competent and an experienced writer with impeccable research and analytical skills. I am capable of producing quality content promptly. My core specialty includes health and medical sciences, but I can competently handle a vast majority of disciplines.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Boogie and Chacha Co. reported a net income of Php430,000 for the first year of its operations. Partner salary allowances are Boogie Php160,000 and Chacha Php120,000. Indicate the division of net...
-
One of the reactions that occurs in a blast furnace, where iron ore is converted to cast iron, is Suppose that 1.64 Ã 103 kg of Fe are obtained from a 2.62 Ã 103-kg sample of Fe2O3....
-
One of the chemical properties that make cyclobutadiene difficult to isolate is that it reacts readily with itself to give a dimer: What reaction of dienes does this resemble? I
-
F udy A big part of communication is sharing personal information with another person. Some information we believe we have the right to be our own and should remain private. However, the degree to...
-
On February 5, Devon entered into a written agreement with Gordon whereby Gordon agreed to drill a well on Devons property for the sum of $5,000 and to complete the well on or before April 15. Before...
-
How is capital budgeting similar to security valuation? How is it different?
-
What does it mean to assert that the delta of a call option is 0.7? How can a short position in 1,000 options be made delta neutral when the delta of a long position in each option is 0.7?
-
Frederick Manufacturing Corp. ordered 500 dozen units of Import Traders rubber pads for $2,580. The order indicated that the pads should be as soft as possible. Import Traders delivered the rubber...
-
gogo Company has the following standards for its single product: standard quantity standard price direct materials 12 pounds per unit $4.25 per pound direct labor 8 hours per unit $14.00 per hour...
-
Clampett Oil purchases crude oil products from suppliers in Texas (TX), Oklahoma (OK), Pennsylvania (PA), and Alabama (AL), from which it refines four end-products: gasoline, kerosene, heating oil,...
-
Estimate the standard enthalpies of formation at 25 C and 1 bar of (a) OH(g); (b) N 2 H 4 (g). Write Lewis structures and use data from Table 10.3, as necessary. Table 10.3 TABLE 10.3 Some Average...
-
Use data from Table 10.3 to estimate the enthalpy change ( r H) for the following reaction. Table 10.3 CH6(g) + Cl2(g) CH5Cl(g) + HCl(g) AH = ?
-
Is Stakeholder Theory a single well-defined theory or a broad umbrella term for a whole lot of different theories? Explain your answer.
-
At the beginning of the period, the Grinding Department budgeted direct labor of $171,200 and property tax of $57,000 for 10,700 hours of production. The department actually completed 12,800 hours of...
-
The following information is available for Shamrock Corporation for the year ended December 31, 2025. Beginning cash balance $ 58,500 Accounts payable decrease 4,810 Depreciation expense 210,600...
-
In today's stock market, compounding is the key to making money in the future for one's investments. However, with decentralized currency growing rapidly (Crypto), how can one rely on TVM for FV...
-
Contract for construction crew and equipment 8 Build parking lots Exterior lighting 11 7 20 12 Build foundation Start Interior Interior 12 9 electrical Final wiring finish Purchase 8 14 12 material...
-
Mad Hatter Enterprises purchased new equipment for $369,000, terms f.o.b. shipping point. Other costs connected with the purchase were as follows: State sales tax Freight costs Insurance while in...
-
Write an equation for the reaction, if any, between a. p-nitrophenol and aqueous potassium hydroxide b. Cyclohexanol and aqueous potassium hydroxide
-
Rowland Textile Inc. manufactures two products: sweatshirts and T-shirts. The manufacturing process involves two activities: cutting and sewing. Expected overhead costs and cost drivers are as...
-
Calculate the NPV and rate of return for each of the following investments. The opportunity cost of capital is 20 percent for all four investments. a. Which investment is most valuable? b. Suppose...
-
In Section 2.1, we analyzed the possible construction of an office building on a plot of land appraised at $50,000. We concluded that this investment had a positive NPV of $7,143 at a discount rate...
-
Explain why the discount rate equals the opportunity cost of capital.
-
With a 35 percent marginal tax rate, would a tax-free yield of 6.1 percent or a taxable yield of 10 percent give you a better return on your savings?
-
3 fiancial factors of singpore post limited during 2015 to 2020
-
Hello! Please help me answer this financial question: 1) A portfolio manager adds a new stock that has the same standard deviation of return as the existing portfolio but has a correlation...

Study smarter with the SolutionInn App


