Use data from Table 10.3 to estimate the enthalpy change ( r H) for the following reaction.
Question:
Use data from Table 10.3 to estimate the enthalpy change (ΔrH) for the following reaction.
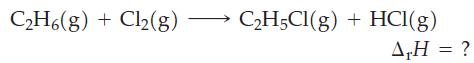 Table 10.3
Table 10.3
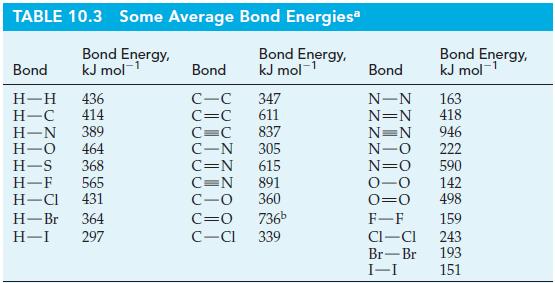
Transcribed Image Text:
C₂H6(g) + Cl2(g) C₂H5Cl(g) + HCl(g) AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
C2H6 Cl2g CHC HClg AH ...View the full answer

Answered By

Antony Sang
I am a research and academic writer whose work is outstanding. I always have my customer's interests at heart. Time is an important factor in our day to day life so I am always time conscious. Plagiarism has never been my thing whatsoever. I give best Research Papers, Computer science and IT papers, Lab reports, Law, programming, Term papers, English and literature, History, Math, Accounting, Business Studies, Finance, Economics, Business Management, Chemistry, Biology, Physics, Anthropology, Sociology, Psychology, Nutrition, Creative Writing, Health Care, Nursing, and Articles.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use Table 8.4 to estimate the enthalpy change for each of the following reactions: a. H2C == O (g) + HCl (g) H3C - O - Cl (g) b. H2O2 (g) + 2CO (g) H2 (g) + CO2 (g) (c). 3H2C == CH2 (g) C6H12 (g)...
-
Ammonia is produced directly from nitrogen and hydrogen by using the Haber process. The chemical reaction is N2 (g) + 3H2 (g) 2NH3 (g) (a) Use Table 8.4 to estimate the enthalpy change for the...
-
(a) Use bond enthalpies to estimate the enthalpy change for the reaction of hydrogen with ethylene: H2 (g) + C2H4 (g) C2H6 (g) (b) Calculate the standard enthalpy change for this reaction, using...
-
Education is a very important job because it can change and shape people's lives. It gives people the knowledge, skills, and attitudes they need to be successful in their personal and work lives. As...
-
The Snyder Mfg. Co., being a large user of coal, entered into separate contracts with several coal companies. In each contract it was agreed that the coal company would supply coal during the entire...
-
Adamson Corporation is considering four average-risk projects with the following costs and rates of return: The company estimates that it can issue debt at a rate of r d = 10%, and its tax rate is...
-
The gamma of a delta-neutral portfolio is 30 (per $ per $). Estimate what happens to the value of the portfolio when the price of the underlying asset (a) suddenly increases by $2 and (b) suddenly...
-
Thirteen students entered the business program at Hillcrest College 2 years ago. The following table indicates what each student scored on the high school SAT math exam and their grade-point averages...
-
Calculator A legal document that indicates the name of the issuer, the face value of the bond and such other data is called a trading on the equity Ob a convertible bond c. a bond debenture Rd. a...
-
You, CA, are the audit senior of Ball Construction Corporation (BC), a small public company. It is September 19, 2013, and the year-end audit fieldwork has just been completed. The audit partner,...
-
One of the chemical reactions that occurs in the formation of photochemical smog is O 3 + NO NO 2 + O 2 . Estimate r H for this reaction by using appropriate Lewis structures and data from Table...
-
A reaction involved in the formation of ozone in the upper atmosphere is O 2 2 O. Without referring to Table 10.3, indicate whether this reaction is endothermic or exothermic. Explain. Table 10.3...
-
Frogue Corporation uses a standard cost system. The following information was provided for the period that just ended: Actual price per kilogram...
-
Write down a C program that takes runs scored by a batsman and prints the status according to the following policy: Runs scored >80 50-79 30-49 10-29 <10 Grade Excellent 4 Very Good Good Average Poor
-
Consider the standard two-period maximization problem for investor j over s states of nature: Subject to S max u(c) + (s)u(c;}(s)) S=1 Cjo + q(s) C; (s) = Wjo +244) S=1 where all terms are as defined...
-
At what point should a leader cease gathering data, take the risk, and simply make the decision? Support your position.
-
In the exchange lemma for the scheduling problem, we say that the first event to finish a* in a given time period [i,j] is always part of the optimal solution for that same time period. To argue...
-
4 10 points Company's year-end is December 31. Calculate depreciation for each year of the machine's estimated useful life under each of the following methods: (Do not round intermediate...
-
Write the equation for the reaction of t-butyl alcohol with potassium metal. Name the product.
-
1. Using the information from Problem 16-4B, prepare a statement of cash flows for Lim Garden Supplies Inc. using the direct method of presenting cash flows from operating activities. 2. How does Lim...
-
Norman Gerrymander has just received a $2 million bequest. How should he invest it? There are four immediate alternatives. a.Investment in one-year U.S. government securities yielding 5 percent. b. A...
-
Show that your answers to Practice Question 7 are consistent with the rate of return rule for investment decisions. a.Investment in one-year U.S. government securities yielding 5 percent. b. A loan...
-
Take another look at investment opportunity (d) in Practice Question 7. Suppose a bank offers Norman a $600,000 personal loan at 8 percent. (Norman is a long-time customer of the bank and has an...
-
i just need anssers for G,h1,h2,h3 120 a. If the opportunity cost of capital is 11%, which of these two projects would you accept (A, B, or both)? b. Suppose that you can choose only one of these two...
-
In using Verizon Communications Inc as a case analysis, what is their product portfolio, competitors and competitive Environment?/
-
Tony and Suzie graduate from college in May 2021 and begin developing their new business. They begin by offering clinics for basic outdoor activities such as mountain biking or kayaking. Upon...

Study smarter with the SolutionInn App


