A reaction involved in the formation of ozone in the upper atmosphere is O 2 2
Question:
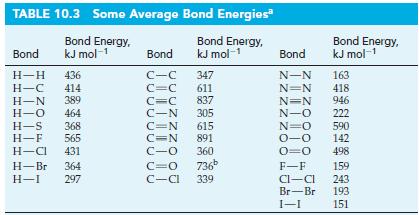
A reaction involved in the formation of ozone in the upper atmosphere is O2 → 2 O. Without referring to Table 10.3, indicate whether this reaction is endothermic or exothermic. Explain.
Table 10.3
Transcribed Image Text:
TABLE 10.3 Some Average Bond Energies Bond Energy, kJ mol-1 Bond Energy, kJ mol-1 Bond H-H 436 H-C 414 H-N 389 H-O 464 H-S 368 H-F 565 H-Cl 431 H-Br 364 H-I 297 Bond C-C C=C 347 611 837 C=C C-N 305 C=N 615 C=N 891 360 C-O C=O 736⁰ C-Cl 339 Bond Energy, kJ mol-1 Bond N-N 163 N=N 418 N=N 946 N-O 222 N=O 590 0-0 142 0=0 498 F-F Cl-Cl Br-Br 1-1 159 243 193 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
To determine whether the reaction O2 2 O is endothermic or exotherm...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A reaction involved in the metabolism of sugars is the splitting of fructose-1,6-diphosphate to give glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. In the living system, this retro-aldol...
-
Write sentences for the long-term direction and strategic path that management intends to follow. "Where we are headed?" and should explain why the direction in which you intend to point the company...
-
Explain what energy terms are involved in the formation of an ionic solid from atoms. In what way should these terms change (become larger or smaller) to give the lowest energy possible for the solid?
-
An important U.S. government organization charged with setting human resource management guidelines is O the EEOC (Equal Employment Opportunity Commission). the OSHA (Occupational Safety and Health...
-
George owed Keith $800 on a personal loan. Neither the amount of the debt nor Georges liability to pay the $800 was disputed. Keith had also rendered services as a carpenter to George without any...
-
The Bouchard Companys EPS was $6.50 in 2021, up from $4.42 in 2016. The company pays out 40% of its earnings as dividends, and its common stock sells for $36.00. a. Calculate the past growth rate in...
-
The vega of a derivatives portfolio dependent on the USD/GBP exchange rate is 200 ($ per %). Estimate the effect on the portfolio of an increase in the volatility of the exchange rate from 12% to 14%.
-
Travelcraft, Inc., manufactures a complete line of fiberglass suitcases and attach cases. The firm has three manufacturing departments: Molding, Component, and Assembly. There are also two service...
-
Current Attempt in Progress Joe Schreiner, controller for Culver Company Inc., recently prepared the company's income statement and statement of changes in equity for 2020. Schreiner believes that...
-
Snow Corporation acquired all of the outstanding $10 par voting common stock of Lannister, Inc., on January 1, Year 2, in exchange for 50,000 shares of its $10 par voting common stock. On December...
-
Use data from Table 10.3 to estimate the enthalpy change ( r H) for the following reaction. Table 10.3 CH6(g) + Cl2(g) CH5Cl(g) + HCl(g) AH = ?
-
Write a Lewis structure of the hydroxylamine molecule, H 2 NOH. Then, with data from Table 10.2, determine all the bond lengths. Table 10.2 TABLE 10.2 Some Average Bond Lengthsa Bond Length, pm 74.14...
-
Lean is a performance improvement strategy that emphasizes reducing waste, with waste defined as activity that adds less value than it costs. Examples of waste include patients waiting time (which...
-
3. The walls of an oven are made from steel sheets with insulating board between them of thermal conductivity 0.18 J m-1 s -1 C-1 . If the maximum internal temperature in the oven is 300C and the...
-
Egyptian Spa produces two different spa products: Relax and Refresh. The company uses three operations to manufacture the products: mixing, blending, and packaging. Because of the materials used,...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
The balances of selected accounts of Casper Company on February 28, 20X1, were as follows: Sales $250,000 and Sales Returns and Allowances $4,000. The firm's net sales are subject to an 7 percent...
-
1. Draw and label force diagrams for the physics book and for the calculator. Add equality marks showing any equalities between force diagrams. Circle and label any Newton's third law pairs. (6 pts)...
-
Rank the following five compounds in order of increasing acid strength: 2-chloroethanol, p-chlorophenol, p-methylphenol, ethanol, and phenol.
-
When the Department of Homeland Security created a color-coded system to prepare government officials and the public against terrorist attacks, what did it do right and what did it do wrong?
-
Respond to the following comments. a. My companys cost of capital is the rate we pay to the bank when we borrow money. b. Net present value is just theory. It has no practical relevance. We maximize...
-
Ms. Smith is retired and depends on her investments for retirement income. Mr. Jones is a young executive who wants to save for the future. They are both stockholders in Airbus, which is investing...
-
Answer this question by drawing graphs like Figure. Casper Milk toast has $200,000 available to support consumption in periods 0 (now) and 1 (next year). He wants to consume exactly the same amount...
-
A total of 2,000 units of Product A are produced from a joint process. Product A can be sold at the split-off point for $16 per unit, or it can be processed further for an additional total cost of...
-
How has COVID - 19 affected the job market in Canada Summary of all research conducted with a minimum of 3 credible sources? An activity you will be using to engage the audience and enforce the...
-
please as soon as can i will rate the thumps up IF THE BANK GIVES YOU 5% INTEREST ON YOUR SAVING ACCOUNT AND INFLATION IS 3% WHAT IS YOU Select one: O a. CANNOT BE CALCULATED b. 1.94% O c. 2% O d....

Study smarter with the SolutionInn App


