For each of the following ions, write two equationsone showing its ionization as an acid and the
Question:
For each of the following ions, write two equations—one showing its ionization as an acid and the other as a base:
(a) HSO3-;
(b) HS-;
(c) HPO4-.
Then use data from Table 16.5 to predict whether each ion makes the solution acidic or basic.
Table 16.5
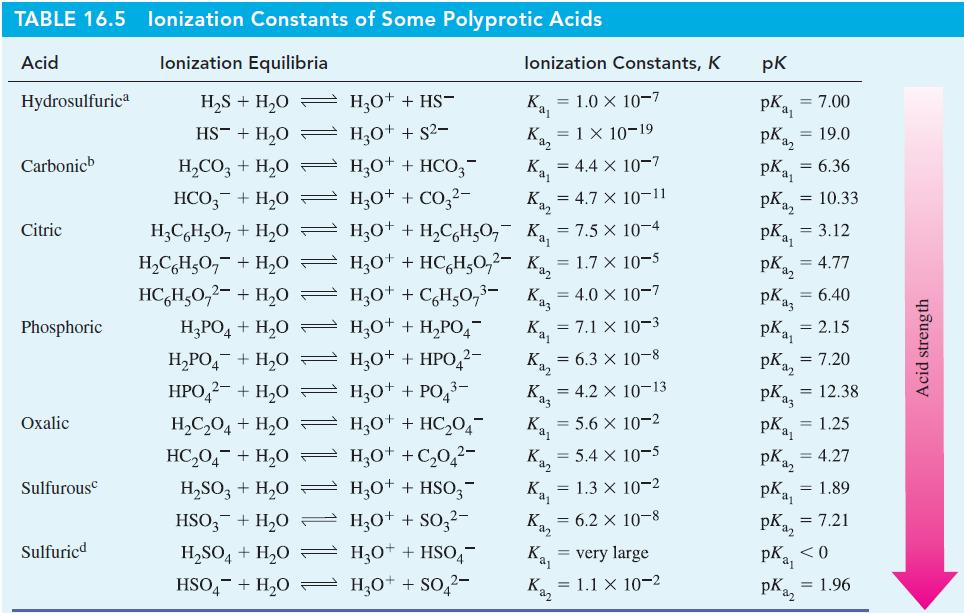
Transcribed Image Text:
TABLE 16.5 lonization Constants of Some Polyprotic Acids Acid lonization Equilibria Hydrosulfurica Carbonicb Citric Phosphoric Oxalic Sulfurousc Sulfuricd H₂S + H₂O HS + H₂O H₂CO3 + H₂O HCO3 + H₂0 H₂PO4+H₂O H₂PO4¯ + H₂0 — HPO42- + H₂O → H₂C₂O4 + H₂0 HC,O4 + H,O H₂SO3 + H₂O — HSO3 + H₂0 H₂O+ + HS¯ H₂O+ + S²- — H₂0+ + HCO3- H3O+ + CO3²- Kaj K₁₂ = 4.7 x 10-11 H₂CH₂O + H₂O H₂O+ + H₂CH₂O₂K₁₁ = 7.5 × 10-4 H₂CH₂O₂ + H₂O H₂O+ + HC6H₂0,²- K₁₂ = 1.7 x 10-5 HC₂H₂0₂² + H₂0 — H3O+ + C¿H²0,³- Kaz = 4.0 x 10-7 K₁₁ = 7.1 × 10-3 K₁₂ = 6.3 × 10-8 = 4.2 X 10-13 Kaz Ka₁ = 5.6 x 10-2 Kaz H₂O+ + H₂PO4¯ H3O+ + HPO ²− H₂0+ + PO4³- H30+ + HC₂04- H₂O+ + C₂0₂²- H3O+ + HSO3- H3O+ + SO3²- lonization Constants, K = 1.0 × 10-7 H₂SO4+H₂O → H3O+ + HSO4- HSO4+H₂O H₂O+ + SO4²- Kaj Kaz Kay Kaz = 1 × 10-19 Kaz = 4.4 x 10-7 = 5.4 x 10-5 = 1.3 x 10-2 = 6.2 × 10-8 Kaj very large = 1.1 x 10-2 pk pka₁ = 7.00 pka2 pkal pka2 = 10.33 PK₁₁ = 3.12 PK₁₂ = 4.77 = 19.0 = 6.36 pK₁3 = 6.40 pK₁₁ = 2.15 PK₁₂ = 7.20 pk az pka₁ pKaz = 12.38 pK₁₁ = 1.25 pka₂ = 4.27 pka₁ = 1.89 pka₂ = 7.21 <0 = 1.96 Acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a HSO3 Acid ionization HSO3 H2O H3O SO3 Base ionization HSO3 H2O HS OH According to T...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
For each of the following brief scenarios, assume that you are reporting on a clients financial statements. Reply as to the type(s) of opinion (per below) possible for the scenario. In addition: ...
-
If the appropriate discount rate for the following cash flows is 7.13 percent per year, what is the present value of the cash flows? Year Cash Flow 1 ......................$1,400 2...
-
Find the conversion (or stock) value for each of the $1,000-par-value convertible bonds described in the following table. Current market price of stock $42.25 50.00 44.00 19.50 Conversion Convertible...
-
A population has a mean of 200 and a standard deviation of 50. Suppose a simple random sample of size 100 is selected and is used to estimate . a. What is the probability that the sample mean will be...
-
Why would evaluating whether a project achieved its MOV make many project managers and teams anxious? Why should it still be done? AppendixLO1
-
1. Discuss the factors that may cause Jane to intentionally and unintentionally distort her ratings of Barb and George. 2. Evaluate the kinds of training programs that could help minimize the factors...
-
Jarett Motors is trying to decide whether it should keep its existing car washing machine or purchase a new one that has technological advantages (which translate into cost savings) over the existing...
-
What is the pH of an aqueous solution that is 0.123 M NH 4 Cl?
-
What is the pH of an aqueous solution that is 0.089 M NaOCl?
-
On January 1, 2019, Arch Ltd. purchased 30% of the common shares of AP Inc. for $1,700,000. In 2019, AP reported net income of $880,000 and paid dividends of $600,000.
-
The accounting records of the Eco Paper Company include the following information relating to the current year ended 31 March 2023: Materials 31 March 2023 $20,000 1 April 2022 $25,000 Work in...
-
The first read is an article on the development of money of a World War II prisoner-of-war, which was published in 1945. The second article was published in the opinion section of the New York Times...
-
Describe each Speaker's basic assumptions regarding employee motivation. That is, what are the underlying principles which guide how the Speaker treats his/her people (i.e., their direct report...
-
Find the area of the shaded region. The graph to the rate of IQ scores of adults, and those scores are normally distributed with the mean of 100 and a standard deviation of 15. x=81
-
In which scenario is Nikki showing resilience to stress? Nikki lost her job as an engineer 3 months ago. At first, she was depressed, but she realized she wanted to change career paths and decided to...
-
On October 31, the stockholders' equity section of Manolo Company's balance sheet consists of common stock $648,000 and retained earnings $400,000. Manolo is considering the following two courses of...
-
Why is inventory management important for merchandising and manufacturing firms and what are the main tradeoffs for firms in managing their inventory?
-
Chalet Sports sells hunting and fishing equipment and provides guided hunting and fishing trips. Chalet Sports is owned and operated by Cliff Owen, a well-known sports enthusiast and hunter. Cliffs...
-
The total assets and total liabilities of Coca-Cola and PepsiCo are shown below. Determine the owners' equity of each company. Coca-Cola (in millions) Pepsico (in millions) Assets Liabilities $29,963...
-
The total assets and total liabilities of eBay and Google are shown below. Determine the owners equity of eachcompany. Google (in millions) eBay (in millions) $18,473 1,433 Assets Liabilities $13,494...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
U Brands Magnetic Dry Erase Board 35 X 35 Inches Black Aluminum Frame - ISBN: B07JC8Y53J - Free Book

Study smarter with the SolutionInn App


