Question: For the reversible reaction A + B C + D, the enthalpy change of the forward reaction is +21 kJ/mol. The activation energy of
For the reversible reaction A + B ⇌ C + D, the enthalpy change of the forward reaction is +21 kJ/mol. The activation energy of the forward reaction is 84 kJ/mol.
(a) What is the activation energy of the reverse reaction?
(b) In the manner of Figure 20-10, sketch the reaction profile of this reaction.
Figure 20-10
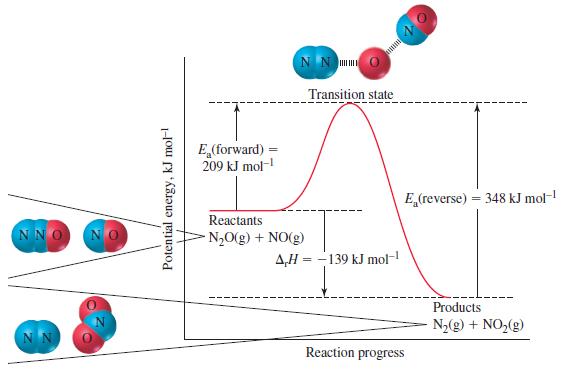
NNO Potential energy, kJ mol- E (forward) 209 kJ mol- NN Reactants NO(g) + NO(g) Transition state AH-139 kJ mol- E (reverse) = 348 kJ mol- Reaction progress Products N(g) + NO(g)
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


