At what temperature will the rate constant for the reaction in Exercise 51 have the value k
Question:
At what temperature will the rate constant for the reaction in Exercise 51 have the value k = 5.0 x 10-3 M-1 s-1?
Exercise 51
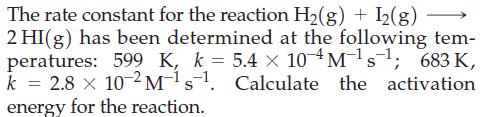
Transcribed Image Text:
The rate constant for the reaction H₂(g) + I2(g) 2 HI(g) has been determined at the following tem- peratures: 599 K, k = 5.4 x 10M¹s¹; 683 K, k 2.8 x 10-2 M¹s¹. Calculate the activation energy for the reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The Arrhenius equation relates the rate constant k of a reaction to the t...View the full answer

Answered By

ZIPPORAH KISIO LUNGI
I have worked on several other sites for more than five years, and I always handle clients work with due diligence and professionalism. Am versed with adequate experience in the fields mentioned above in which have delivered quality papers in research, thesis, essays, blog articles, and so forth.
I have gained extensive experience in assisting students to acquire top grades in biological, business and IT papers. Notwithstanding that, I have 7+ years of experience in corporate world software design and development.
5.00+
194+ Reviews
341+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
25% of available points for each transaction, indicate the impact on total assets, total liabilities and total equity. Enter decreases as negative values. If there is no impact, either enter a 0, or...
-
The purpose of ERISA is to create transparency, accountability, and prevent the mismanagement of the investments made by participants in Plans. This week we are examining the requisite requirements...
-
The reaction NO(g) + O3(g) NO2(g) + O2(g) was studied by performing two experiments. In the first experiment (results shown in following table), the rate of disappearance of NO was followed in a...
-
Identify an accurate sentence about parenting in the United States. a. Fathers of toddlers play more roughly with daughters than with sons. b. During the first year, fathers treat boys and girls as...
-
The company signed an $800,000 contract to build an environmentally friendly access trail to South Willow Lake. The project was expected to take approximately three years. The following information...
-
Obtain the state model for the reduced-form model 2 + 5 + 4x = 4y(t).
-
Calculate r2 for the least squares line in Exercise 11.21 LO9 (p. 625).
-
The following are several of Graf Corporations accounts at the end of 2016: Account Credit Balance Common Stock, $10 par............. $ 47,100 Bonds Payable (due 2017)............ 126,000 Additional...
-
Serial Problem Business Solutions LO P1, P2, P3, P4, P5, P6 After the success of the companys first two months, Santana Rey continues to operate Business Solutions. The November 30, 2019, unadjusted...
-
The first-order reaction A products has a half-life, t 1/2 , of 46.2 min at 25C and 2.6 min at 102C. (a) Calculate the activation energy of this reaction. (b) At what temperature would the half-life...
-
For the reversible reaction A + B C + D, the enthalpy change of the forward reaction is +21 kJ/mol. The activation energy of the forward reaction is 84 kJ/mol. (a) What is the activation energy of...
-
Which of the specific environmental and organizational HR challenges identified in this chapter will be most important in healthcare in the next 20 years? Use your own experience in your answer.
-
Research a company that declared a 100% stock dividend or a two-for-one split Contrast the differences between a stock dividend and a stock split. Imagine that you are a stockholder in a company....
-
What are your ideas for Implementation and Assessing the Solution? How did you implement and assess the success? What should the time frame look like? What resources will be needed? What criteria...
-
What is an aesthetic question a viewer might ask about a work of art? 1 . What principles of design were used to make this work? 2 . What qualifies a functional object like this as a work of art? 3 ....
-
2. A phase diagram is shown below for an allotropic metal. Sketch and label possible Gibbs free energy curves for the 3 phases, as a function of temperature for the pressure indicated. Does the a or...
-
Recommend at least one (1) way a business with which you are familiar could use social media / buzz marketing to increase sales and awareness (e.g., social media awareness) of your...
-
Mobile telephones perform handoffs as they move from cell to cell. During a call, a telephone either performs zero handoffs (H0), one handoff (H1), or more than one handoff (H2). In addition, each...
-
Michelles trust is subject to 3.8% surtax on the lesser of the trusts net investment income or the excess of the trusts adjusted gross income over the $12,400 threshold (the highest trust tax rate)....
-
Galindo Co. uses special journals and a general journal. Identify the journal in which each of the following transactions is recorded. (a) Purchased equipment on account. (b) Purchased merchandise on...
-
Identify the special journal(s) in which the following column headings appear. 1. Sales Discounts Dr. 2. Accounts Receivable Cr. 3. Cash Dr. 4. Sales Cr. 5. Merchandise Inventory Dr.
-
Kidwell Computer Components Inc. uses a multi-column cash receipts journal. Indicate which column(s) is/are posted only in total, only daily, or both in total and daily. 1. Accounts Receivable 2....
-
explain the concept of Time Value of Money and provide and example. In addition to your discussion, please explain the differences between Stocks and Bonds
-
Wildhorse Inc. has just paid a dividend of $3.80. An analyst forecasts annual dividend growth of 9 percent for the next five years; then dividends will decrease by 1 percent per year in perpetuity....
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...

Study smarter with the SolutionInn App


