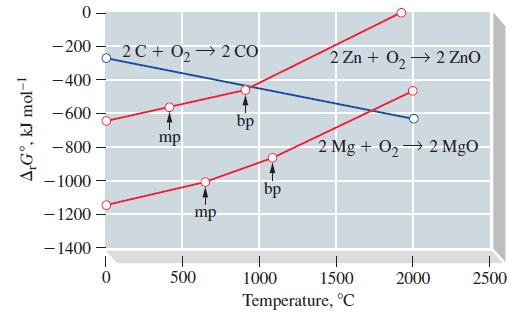
Question: For the straight-line graphs in Figure 23-8, explain why (a) Breaks occur at the melting points and boiling points of the metals; (b) The slopes
For the straight-line graphs in Figure 23-8, explain why
(a) Breaks occur at the melting points and boiling points of the metals;
(b) The slopes of the lines become more positive at these breaks;
(c) The break at the boiling point is sharper than at the melting point.
Figure 23-8
A,G, kJ mol-1 -200 - 400 -600 -800 - 1000 - 1200 - 1400 | 0 2C+0 2 CO mp mp 500 bp bp 2 Zn + 02 ZnO 2 Mg + O 2 MgO 1000 1500 Temperature, C 2000 2500
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
a Breaks occur at the melting points and boiling points of the metals because these points represent ... View full answer

Get step-by-step solutions from verified subject matter experts


