For which of the following reactions would you expect the extent of the forward reaction to increase
Question:
For which of the following reactions would you expect the extent of the forward reaction to increase with increasing temperatures? Explain.
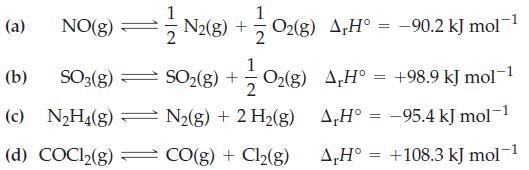
Transcribed Image Text:
(a) NO(g) =// N2(8) + O₂(8) A‚H° = −90.2 KJ mol¯ O₂(g) A,H° +98.9 kJ mol-1 A,H° = -95.4 kJ mol-1 A,Hº +108.3 kJ mol-1 (b) SO3(g) (c) N₂H4(g) (d) COC1₂(g) = SO₂(g) + 2 N₂(g) + 2 H₂(g) N₂(g) + 2 H₂(g) CO(g) + Cl₂(g) = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
To determine which of the given reactions would have an increase in the extent of the forward reacti...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Which of the following reactions would you expect to have the larger rate at room temperature? Why? (Hint: Think of which would have the lower activation energy.) 2Ce4+(aq) + Hg22+(aq) 2Ce3+(aq) +...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
(a) In which of the following reactions would you expect the orientation factor to be least important in leading to reaction: NO + O NO2 or H + CI HCI? (b) How does the kinetic-molecular theory...
-
Transactions related to revenue and cash receipts completed by Acheville Architects Co. during the period September 2-30, 2014, are as follows: Sept. 2. Issued Invoice No. 793 to Nickle Co., $5,200....
-
What benefit is available to participants in a dividend reinvestment plan? How might the firm benefit?
-
XYZ Company is a U.S. firm that makes communication software used in a variety of consumer goods manufactured and sold in the United States. XYZ recently learned that one of the manufacturing firms...
-
3. Describe the benefits of a scenario DCF valuation model. What factors should be considered when constructing scenario parameters?
-
Dunlop Company makes a product that it sells for $200. Dunlop incurs annual fixed costs of $250,000 and variable costs of $160 per unit. Required The following requirements are interdependent. For...
-
1.Explain the difference between authorized shares and outstanding shares. 2. What is the difference between cumulative preferred shares and non-cumulative preferred shares? Which situation could...
-
The following reaction represents the binding of oxygen by the protein hemoglobin (Hb): Explain how each of the following affects the amount of Hb:O 2 : (a) Increasing the temperature; (b) Decreasing...
-
What effect does increasing the volume of the system have on the equilibrium condition in each of the following reactions? (a) C(s) + HO(g) = CO(g) + H(g) (b) Ca(OH)2(s) + CO2(g) CaCO3(s) + HO(g) (c)...
-
Without doing any computation, decide which has a higher probability, assuming each sample is from a population that is normally distributed with = 100 and = 15. Explain your reasoning. (a) P(90 x...
-
Manufacturing company produces $3800 worth of products weekly. If the cost of raw materials to make this product is $400, and the labour cost is $360, calculate the productivity.
-
1-You are a very well-recognized professional in your area, with many years of experience solving international conflicts. There is a company in the middle of two European countries that are fighting...
-
Find the solution u = u(x,y) of the following problem on the set R. u du - 4, (1.4) Ju(0,y) =3y, u(x, 0) = 0. (1.5) ay
-
Scenario A Sports Club 10 Highfield Sports Club has organised a fundraising event. 300 tickets have been sold at a price of $2.50 each. Money taken at the event Percentage of money (E) taken (96)...
-
Shamrock Investments has three divisions (Green, Clover, Seamrog) organized for performance evaluation purposes as investment centers. Each division's required rate of return for purposes of...
-
Depreciation information for Gordon Chemicals Company is given in BE9-4. Assuming the declining-balance depreciation rate is double the straight-line rate, compute annual depreciation for the first...
-
The Place-Plus real estate development firm in Problem 24 is dissatisfied with the economists estimate of the probabilities of future interest rate movement, so it is considering having a financial...
-
Give an example of how the capital expenditures budget affects other operating budgets
-
At the beginning of the period, the Fabricating Department budgeted direct labor of $22,500 and equipment depreciation of $7,000 for 900 hours of production. The department actually completed 750...
-
At the beginning of the period, the Assembly Department budgeted direct labor of $186,000 and property tax of $15,000 for 12,000 hours of production. The department actually completed 13,400 hours of...
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


