What effect does increasing the volume of the system have on the equilibrium condition in each of
Question:
What effect does increasing the volume of the system have on the equilibrium condition in each of the following reactions?
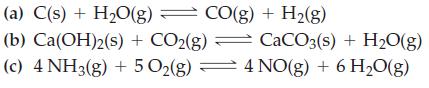
Transcribed Image Text:
(a) C(s) + H₂O(g) = CO(g) + H₂(g) (b) Ca(OH)2(s) + CO2(g) CaCO3(s) + H₂O(g) (c) 4 NH3(g) + 5 O₂(g) 4 NO(g) + 6 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Increasing the volume of the system has a different effect on the equilibrium condition of each reac...View the full answer

Answered By

William Otieno
I am a professional tutor and a writer with excellent skills that are important in serving the bloggers and other specialties that requires a great writer. The important aspects of being the best are that I have served so many clients with excellence
With excellent skills, I have acquired very many recommendations which have made it possible for me to survive as an excellent and cherished writer. Being an excellent content writer am also a reputable IT writer with essential skills that can make one turn papers into excellent result.
4.70+
83+ Reviews
354+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(a) Use the reaction quotient to determine the direction the reaction must proceed to reach equilibrium. (b) Calculate the equilibrium partial pressures of the gases. (c) What effect will increasing...
-
What effect does increasing the required return have on the present value of a future amount? Why?
-
Submit the completed Marketing Math Calculations worksheet.Also, please submit a separate Word document in which you answer the following questions: If the retail price is set at $1.00, what effect...
-
Prepare journal entries for each of the following transactions: 1. Purchase equipment in exchange for cash of $22,400. 2. Provide services to customers and receive cash of $5,100. 3. Pay the current...
-
What effect did the Jobs and Growth Tax Relief Reconciliation Act of 2003 have on the taxation of corporate dividends? On corporate dividend payouts?
-
Hello-Hello is a U.S. telecommunications company with global operations. Sophia is an assistant vice president of Hello-Hello. She is dispatched to China to handle two situations. First, a shipment...
-
4. You are computing the value of a firm headquartered in an emerging market. Identify the factors unique to an emergingmarket that need to be evaluated when estimating the cost of equity using the...
-
Mountain Sports Inc. borrowed money for two years last week at 12%. The pure rate is 2%, and Mountains financial condition warrants a default risk premium of 3% and a liquidity risk premium of 2%....
-
Builtrite Nightclubs purchased a disco mirror that currently has a book value of $12,000. If Builtrite sells the disco mirror for $9000 today, then what is the amount of cash that it will net after...
-
For which of the following reactions would you expect the extent of the forward reaction to increase with increasing temperatures? Explain. (a) NO(g) =// N2(8) + O(8) AH = 90.2 KJ mol O(g) A,H +98.9...
-
For the reaction (a) Will K p increase, decrease, or remain constant with temperature? Explain. (b) If a constant-volume mixture at equilibrium at 298 K is heated to 400 K and equilibrium...
-
Define leader and leadership and explain why managers should be leaders.
-
State the vertical asymptotes, if any exist for the function. T f(x) = x+81
-
4. Oh no! Prof. Conlin was doing the dishes, but ran out of space on his drying rack. He decided to set the last two bowls on a towel on the counter to dry. He wondered, "To make sure they get dry,...
-
It has been assumed so far that the firm will operate a project over its full physical life. However, may not be the best option - it may be better to abandon a project prior to the end of potential...
-
33-34 Find (a) f + g, (b) f- g, (c) fg, and (d) f/g and state their domains. 33. f(x)=25-x, g(x) = x+1 ===== 1 34. f(x)= x-1' 9(x)=-2 X
-
Describe five steps independent auditors take when auditing an organization. -An independent auditor, often a public accounting firm, begins an audit by studying the business. This approach helps to...
-
Hunt Company reported net income of $157,000. It reported depreciation expense of $12,000 and accumulated depreciation of $47,000. Amortization expense was $8,000. Hunt purchased new equipment during...
-
Ball bearings are widely used in industrial applications. You work for an industrial food machinery manufacturer and your role is to design the driveshaft assembly on a new type of equipment that...
-
Soft Glow Candle Co. projected sales of 78,000 candles for 2010. The estimated January 1, 2010, inventory is 3,600 units, and the desired December 31, 2010, inventory is 4,500 units. What is the...
-
Day Timer Publishers Inc. projected sales of 205,000 schedule planners for 2010. The estimated January 1, 2010, inventory is 18,500 units, and the desired December 31, 2010, inventory is 15,000...
-
Soft Glow Candle Co, budgeted production of 78,900 candles in 2010. Wax is required to produce a candle. Assume 8 ounces (one half of a pound) of wax is required for each candle. The estimated...
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


