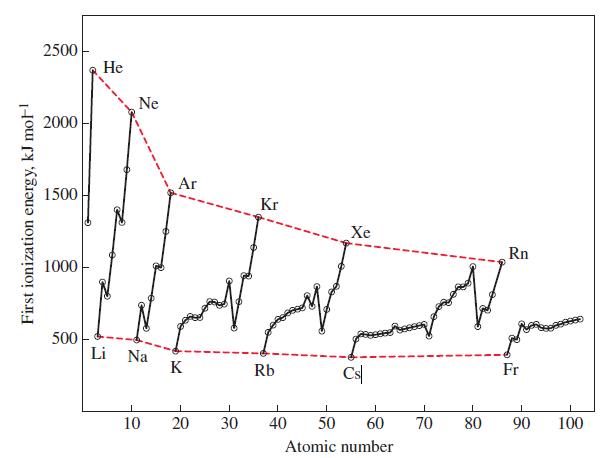
From the data in Figure 9-12, the formation of a gaseous anion Li - appears energetically favorable.
Question:
From the data in Figure 9-12, the formation of a gaseous anion Li- appears energetically favorable. That is, energy is given off when gaseous Li atoms accept electrons. Comment on the likelihood of forming a stable compound containing the Li- ion, such as Li+ Li- or Na+ Li-.
Figure 9-12
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





