From the observation that 0.0500 M vinylacetic acid has a freezing point of -0.096 C, determine K
Question:
From the observation that 0.0500 M vinylacetic acid has a freezing point of -0.096 °C, determine Ka for this acid.
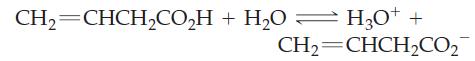
Transcribed Image Text:
CH2=CHCH,CO,H+H,O
CH2=CHCH,CO,H+H,O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the acid dissociation constant Ka of vinylacetic acid from the given information follow ...View the full answer

Answered By

Abigael martinez
I have been a tutor for over 3 years and have had the opportunity to work with students of all ages and backgrounds. I have a strong belief that all students have the ability to learn and succeed if given the right tools and support. I am patient and adaptable, and I take the time to get to know each student's individual learning style in order to best support their needs. I am confident in my ability to help students improve their grades and reach their academic goals.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A 0.500 m solution of MgCl2 has a freezing point of 2.60C. What is the true van't Hoff factor of this ionic compound? Why is it less than the ideal value?
-
A solution of C2H2O4 in CH3COOH has a freezing point of 10.00C. What is the molality of the solution?
-
The following picture represents atoms of hypothetical, nonmetallic, monatomic elements A, B, and C in a container at a temperature of 4 K (the piston maintains the pressure at 1 atm). None of these...
-
Average rates of return on Treasury bills, government bonds, and common stocks, 1900-2020. Average Annual Average Premium (Extra Rate of Return return versus Treasury (8) bills) (%) Portfolio...
-
Willow Enterprises is considering the acquisition of Steadfast Corp. in a stock swap transaction. Currently, Willows stock is selling for $45 per share. Although Steadfasts shares are currently...
-
Why is it important not to view the concept of whistle-blowing as tattle-telling or ratting on another employee?
-
Describe the three tactical approaches to product release or system installation, as well as compare the advantages and disadvantages of each approach: direct cutover parallel phased AppendixLO1
-
Following is a partially completed balance sheet for Epsico, Inc., at December 31, 2010, together with comparative data for the year ended December 31, 2009. From the statement of cash flows for the...
-
A stock has a beta of 1.00, the expected return on the market is 10 percent, and the risk-free rate is 4.10 percent. What must the expected return on this stock be? (Do not round intermediate...
-
You are asked to prepare a 100.0 mL sample of a solution with a pH of 5.50 by dissolving the appropriate amount of a solute in water with pH = 7.00. Which of these solutes would you use, and in what...
-
Explain why [H 3 O + ] in a strong acid solution doubles as the total acid concentration doubles, whereas in aweak acid solution,[H 3 O + ] increases only by about a factor of 2.
-
On January 1, 2010, Valuation Allowance for Available-for-Sale Securities has a credit balance of $3,400. On December 31, 2010, the cost of the available-for-sale securities was $35,700, and the fair...
-
What sutra is used to verify BODMAS principle in Vedic Mathematics?Explain in brief.
-
What Is Accounting? Definition, Types, History, & Examples
-
How Does Accounting Work?
-
What Are the Types of Accounting Practices?
-
The Accounting ProfessionWhat Does an Accountant Do?
-
Moskow Corporation issued the following statement of cash flows for 2017. (a) Compute free cash flow for Moskow Corporation. (b) Explain why free cash flow often provides better information than "Net...
-
Write a function that reads a Float24_t value: Float24_t float24_read(void) A legitimate float24 value string is of the form: "mantissabexponent" where the mantissa (m) and the exponent (e) may have...
-
Boutique Ads Co. produces advertising videos. During the last six months of the current fiscal year, Boutique Ads Co. received the following notes: Instructions1. Determine for each note (a) The due...
-
The following data relate to notes receivable and interest for Vidovich Co Mar. 3. Received a $72,000, 9%, 60-day note on account. 25. Received a $10,000, 8%, 90-day note on account. May 2. Received...
-
The following were selected from among the transactions completed during the current year by Bonita Co., an appliance wholesale company: Jan. 20. Sold merchandise on account to Wilding Co., $30,750....
-
Nitin is paid a base salary of $200 per week and commission at the rate of 3% for sales over $5000, 4% if his sales are over $8000, and 5% if sales are over $15,000. How much will Nitin earn in a...
-
Safa is paid a base salary of $1500 per month and a commission of 6% on all sales over $75,000. Last month, Safa's gross salary was $4440. What were her sales for the month? a$149,000 b$124,000...
-
Your regular hourly rate of pay is $15.86, and you are paid double time for all work on weekends and for any time over forty hours per week (Monday to Friday). Calculate your gross earnings for a...

Study smarter with the SolutionInn App


