How many grams of Ag 2 CO 3 are decomposed to yield 75.1 g Ag in this
Question:
How many grams of Ag2CO3 are decomposed to yield 75.1 g Ag in this reaction?
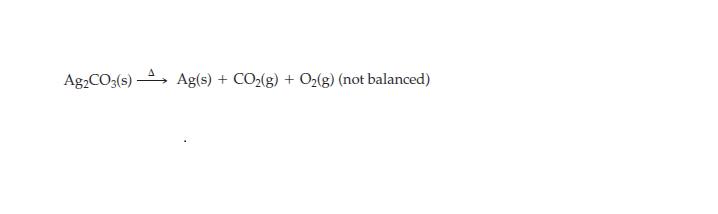
Transcribed Image Text:
Ag2CO3(s) Ag(s) + CO₂(g) + O₂(g) (not balanced)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To solve this problem we need to use stoichiometry First we need to bal...View the full answer

Answered By

John Aketch
I have a 10 years tutoring experience and I have helped thousands of students to accomplish their educational endeavors globally. What interests me most is when I see my students being succeeding in their classwork. I am confident that I will bring a great change to thins organization if granted the opportunity. Thanks
5.00+
8+ Reviews
18+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
Consider the following reaction: (a) Is this reaction exothermic or endothermic? (b) Calculate the amount of heat transferred when 3.55 g of Mg(s) reacts at constant pressure. (c) How many grams of...
-
Consider the following reaction: 2 CH3OH(g) 2 CH4(g) + O2(g).............H = + 252.8 kJ (a) Is this reaction exothermic or endothermic? (b) Calculate the amount of heat transferred when 24.0 g of...
-
The September 30, 2018, adjusted trial balance of Buzzy?s, Inc., is shown next. Requirements 1. Prepare the September closing entries for Buzzy?s, Inc. 2. Calculate the ending balance in Retained...
-
After all revenue and expense accounts have been closed at the end of the fiscal year, Income Summary has a debit of $218,380 and a credit of $375,000. At the same date, Rachel Bray, Capital has a...
-
Sports Biz, a profitable company, built and equipped a $2,000,000 plant brought into operation early in Year 1. Earnings of the company (before depreciation on the new plant and before income taxes)...
-
identify the mainstream of key thoughts in perceptual paths of cultural philosophies,
-
On December 31, Year 1, P Company purchased 80% of the outstanding shares of S Company for $6,960 cash. The statements of financial position of the two companies immediately after the acquisition...
-
'''' Why is adequate planning important in an audit? Please briefly explain the categories of information auditors are required to obtain during the process of "understand the business". With what we...
-
(A) Hexamethylenediamine has the molecular formula C 6 H 16 N 2 . It is one of the starting materials for the production of nylon. It can be prepared by the following reaction: C 6 H 10 O 4 (l) + NH...
-
(a) Determine the stoichiometric numbers for the following reaction, which occurs to a limited extent at 25 C: 4 KO 2 (s) + 2 CO 2 (g) 2 K 2 CO 3 (s) + 3 O 2 (g). (b) In an experiment carried out at...
-
How are financial assets and liabilities held for trading reported on the balance sheet?
-
2. (10 points) Two suppliers of products are available to supply the needs of four supermarkets. Each supplier can provide 90 units per day. Each supermarket would like to receive 60 units per day....
-
QUESTION 3 (11 marks) Midrand Ltd acquired a 90% interest in Bramely Ltd on 2 December 20.21 for R2 million. The consideration was settled as follows: Cash payment, Issue of 100 000 shares to the...
-
1. Prepare a Proforma Income Statement for ACCO 295 Corp. (30 points) Use the same Excel table provided to do the calculations with the class explanation. 1. Selling and administrative expenses were...
-
Sandy Foot Hospital expanded their cardiovascular unit to include more operating rooms. They negotiated a 20-year loan with monthly payments and a large sum of $250,000 due at the end of the loan....
-
Oscillations and Resonance Name Lab Procedure Answer questions in red. Download and run the HTMLS application \"resonance\". Driving force: 30 N Driving equency: 5 rad. '5 Spring constant: 5 - 'Irn...
-
Show how each of the following compounds can be prepared from cyclohexene: a. b. c. d. OH
-
If the amplifier indicated by the box input impedance of oo, which of the following statements are true ? has an open loop gain as well as Feedback factor (\beta = 1/ R_1\) The feedback is voltage...
-
The management of an electronics manufacturing firm believes it is desirable to automate its production facility. The automated equipment would have a l0-year life with no salvage value at the end of...
-
A local symphony association offers memberships as follows: Continuing membership, per year $ 15 Patron lifetime membership 375 The patron membership has been based on the symphony association's...
-
Using capitalized cost determine which type of road surface is preferred on a particular section of highway Use 12% interest rate. A B Initial cost $700,000 $500,000 Annual maintenance Periodic...
-
Gross margin can be found on single step income statement. TRUE FALSE GAAP requires companies to disclose the sales returns and allowance on their Income Statement. TRUE FALSE
-
) Identify how each of the following items is shown on the statement of cash flows. Identify each as operating (O), investing (I), financing (F), or non-cash investing and financing (N). Item (O),...
-
1. Skolnick Corporation has provided the following information: Cost per Unit Cost per Period Direct materials $ 5.50 Direct labor $ 3.30 Variable manufacturing overhead $ 2.10 Fixed manufacturing...

Study smarter with the SolutionInn App


