How much heat, in kilojoules, is associated with the production of 283 kg of slaked lime, Ca(OH)
Question:
How much heat, in kilojoules, is associated with the production of 283 kg of slaked lime, Ca(OH)2?
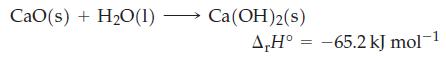
Transcribed Image Text:
CaO(s) + H₂O(1) - Ca(OH)2(s) A,H° -65.2 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To find the heat associated with the production of 283 kg of slaked lime CaOH2 from calcium oxide Ca...View the full answer

Answered By

Oleksandr Kinash
I have a Master's degree in International Economics. I have been working as a tutor and academic research writer for over 5 years now. I mostly help students is Economics, Statistics, and Math.
0.00
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A company is considering a 5-year project that opens a new product line and requires an initial outlay of $77,000. The assumed selling price is $98 per unit, and the variable cost is $60 per unit....
-
Find ATA and AAT. A = [20 3 57 11-3 3 82 0-4 40 0-2 (a) ATA (b) AAT 11
-
What is the classification of the freight in account?
-
A data analyst decided to do all design work from home and wants to create a home office. The analyst needs a new computer for $1,900 and printer/scanner for $250. A vendor offers a financing option...
-
List the four components of a manufacturing statement and provide specific examples of each for Apple.
-
Briefly describe ROLAP architecture.
-
WACC Lancaster Engineering Inc. (LEI) has the following capital structure, which it considers to be optimal: Debt 25% Preferred stock 15 Common equity 60 100% LEIs expected net income this year is...
-
Here are selected transactions for Halverson Corporation for 2012. Jan. 1 Retired a piece of machinery that was purchased on January 1, 2002. The machine cost $47,000 and had a useful life of 10...
-
Pharoah Construction Company uses the percentage - of - completion method of accounting. In 2 0 2 5 , Pharoah began work under a contract with a contract price of $ 1 , 6 5 0 , 0 0 0 . Other details...
-
Prepare the adjusting journal entries in the worksheet labelled ABC. The adjusted trial balance columns will update automatically. You need to enter the journal entry reference letters(a,b,c,etc.) In...
-
What is the final temperature (in C) of 1.24 g of water with an initial temperature of 20.0 C after 6.052 J of heat is added to it?
-
Brass has a density of 8.40 g/cm 3 and a specific heat capacity of 0.385 J g-1 C -1 . A 15.2 cm 3 piece of brass at an initial temperature of 163 C is dropped into an insulated container with 150.0 g...
-
A sample containing 224 88 Ra, which decays by -particle emission, disintegrates at the following rate, expressed as disintegrations per minute or counts per minute (cpm): t = 0, 1000 cpm; t = 1 h,...
-
The following data represent the height of 26 statistics students as measured in inches: a. Create a frequency table for these data. b. Create a histogram for these data with an interval width of 1...
-
Repeat Exercise 15 in Chap. 3 to allow the user to enter temperatures for any number of cities using the best iteration structure. Data From Exercise 15 The dew point temperature is a good indicator...
-
Two stacks of positive integers are needed, one containing elements with values less than or equal to 1,000 and the other containing elements with values larger than 1,000. The total number of...
-
Compare Figures 1-2 and 1-12. How do they differ? How are they similar? Explain how Figure 1-12 conveys the idea of speed in development. Figures 1-2 Figures 1-12 Maintenance Planning Implementation...
-
With a neat sketch explain the working of pressure-velocity compounding of impulse steam turbine.
-
What are some of the techniques that can be used to impart some degree of plasticity to crystalline ceramic materials?
-
Distinguish among total-moisture content, free-moisture content, equilibrium-moisture content, unbound moisture, and bound moisture.
-
Cominsky Company purchased a machine on July 1, 2011, for $28,000. Cominsky paid $200 in title fees and county property tax of $125 on the machine. In addition, Cominsky paid $500 shipping charges...
-
Dickinson Inc. owns the following assets. Compute the composite depreciation rate and the composite life of Dickinson's assets. Estimated Useful Life 10 years Cost Asset Salvage A $70,000 $ 7,000...
-
Holt Company purchased a computer for $8,000 on January 1, 2009. Straight-line depreciation is used, based on a 5-year life and a $1,000 salvage value . In 2011, the estimates are revised. Holt now...
-
Virgil Watson gave his daughter, Holly, a gift of passive activity property. Suspended losses amounted to $30,000 and the property had an adjusted basis of $40,000. Also, the property had a fair...
-
2. Turkey Corp. has a computer that they purchased on March 30, 2016, for $ 106,000. This computer had an estimated life of ten years and a residual value of $ 6,000. On December 31, 2020, the old...
-
Explain the steps in the posting process. Also, discuss what a Trial balance is and why it is an important step in the accounting cycle. Does a trial balance that is in balance guarantee that all...

Study smarter with the SolutionInn App


