How much heat, in kilojoules, is evolved in the complete combustion of (a) 1.325 g C 4
Question:
How much heat, in kilojoules, is evolved in the complete combustion of
(a) 1.325 g C4H10(g) at 25 °C and 1 atm;
(b) 28.4 L C4H10(g) at STP;
(c) 12.6 L C4H10(g) at 23.6 °C and 738 mmHg?
Assume that the enthalpy of reaction does not change significantly with temperature or pressure. The complete combustion of butane, C4H10(g), is represented by the equation
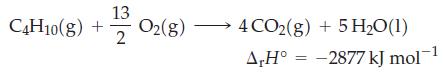
Transcribed Image Text:
C4H10(g) + O₂(g) 13 2 4 CO2(g) + 5H₂0 (1) A,H° -2877 kJ mol™
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To solve this problem we will use the following steps Calculate the moles of butane Mo...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A company is considering a 5-year project that opens a new product line and requires an initial outlay of $77,000. The assumed selling price is $98 per unit, and the variable cost is $60 per unit....
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Randy, Inc., can issue 3-month commercial paper with a face value of $1,500,000 for $1,450,000. Transaction costs will be $1,500. The effective annualized percentage cost of the financing, based on a...
-
Describe the relations among the income statement, the manufacturing statement, and a detailed listing of factory overhead costs.
-
a. Show the dimension EMPLOYEE (with all of its records) if the Type 1 option for handling slowly changing dimensions is applied. b. Show the dimension EMPLOYEE (with all of its records) if the Type...
-
Assume that the risk-free rate increases but the market risk premium remains constant. What impact would this have on the cost of debt? What impact would it have on the cost of equity? AppendixLO1
-
Tom is a CPA for a large regional firm. In preparing the tax return for Espresso Industries, he notices that the firm has an unusually high amount of travel, meal, and entertainment expenses....
-
i need just 4 and 5 requierment Martin Shoes, Inc. manufactures and distributes orthopedic footwear. To sell its products, the marketing department requires sales personnel to call on the shoe...
-
Wiset Company completes these transactions during April of the current year (the terms of all its credit sales are 2/10, n/30). Apr. 2 Purchased $14,300 of merchandise on credit from Noth Company,...
-
The standard enthalpy of reaction for the combustion of octane is rH = -5.48 x 10 3 kJ/mol C 8 H 18 (l). How much heat, in kilojoules, is liberated per gallon of octane burned? (Density of octane =...
-
What is the final temperature (in C) of 1.24 g of water with an initial temperature of 20.0 C after 6.052 J of heat is added to it?
-
The Standards for the Professional Practice of Internal Auditing states that the director of internal auditing should develop schedules which: a. Show what activities are to be audited. b. Are...
-
The Hudson Jewelers case study can be found in Appendix C. Chapter 14 Case Question for Discussion: 1.Customer demand (weekly visits) at Hudson Jewelers is highly seasonal, as shown in the worksheet...
-
Jasmine Minoza, the chief information officer of a Canada- based designer of video games, Adventure Gaming, Inc. (AGI), is considering outsourcing her companys software development activities to...
-
The input to the circuit of Fig. 5-23 with RC = 1 is v 1 = sin t. Write KCL at node B and solve for v 2 . +1 VI R B A + C D 3+ 10-41. 12
-
Draw an angle of 120. First draw a straight line about 6cm long. Place the protractor on the line so that the central cross hair is on one of the end points of the line. Make sure the line lines up...
-
Draw a seriesparallel switch circuit that implements the function f(x, y, z) = 1 if inputs xyz represent either 1 or a prime number in binary (xyz = 001, 010, 011, 101, 111).
-
What is the purpose of the firing or sintering operations in the processing of crystalline ceramic products?
-
For the following exercises, write the first four terms of the sequence. a n = 2 n 2
-
At a recent executive committee meeting, the controller for Ricardo Company remarked, With only a single key difference between U.S. GAAP and iGAAP for property, plant, and equipment, it should be...
-
What is a modified accelerated cost recovery system (MACRS)? Speculate as to why this system is now required for tax purposes.
-
Fernandez Corporation purchased a truck at the beginning of 2010 for $50,000. The truck is estimated to have a salvage value of $2,000 and a useful life of 160,000 miles. It was driven 23,000 miles...
-
Payment of 1 year Insurance policy for $1,500 results in
-
Need help on the balance budgeted sheet little Annin flagmaker liabilities area accounts payable for ending march 31st and June 30th Liabilities Accounts payable $55,000 Little Annin Flagmakers...
-
Assuming an interest rate of 12%, the present value of $70,000 to be received 10 years from now would be closest to: A. $22,540 B. $395,500. C. $84,000 D. $70,000

Study smarter with the SolutionInn App


