In a manner similar to that outlined on page 1022, (a) Write equations to represent the reaction
Question:
In a manner similar to that outlined on page 1022,
(a) Write equations to represent the reaction of (CH3)3SiCl with water, followed by the elimination of H2O from the resulting silanol molecules.
(b) Does a silicone polymer form from part (a)?
(c) What would be the corresponding product obtained from CH3SiCl3?
Transcribed Image Text:
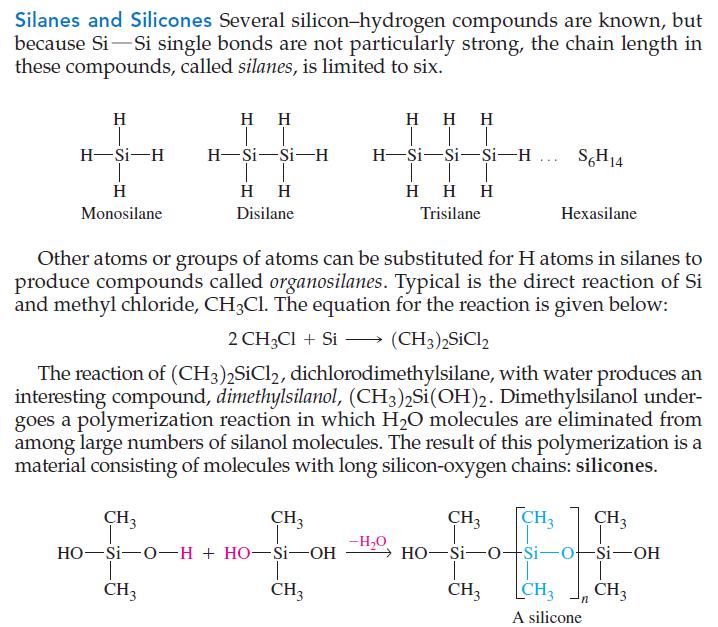
Silanes and Silicones Several silicon-hydrogen compounds are known, but because Si Si single bonds are not particularly strong, the chain length in these compounds, called silanes, is limited to six. H H-Si-H H Monosilane HH T T H-Si-Si-H HH Disilane HHH | T H-Si-Si-Si-H S6H14 CH3 CH3 HO-Si-O-H + HO-Si-OH I CH3 CH3 HHH Trisilane Other atoms or groups of atoms can be substituted for H atoms in silanes to produce compounds called organosilanes. Typical is the direct reaction of Si and methyl chloride, CH3Cl. The equation for the reaction is given below: 2 CH3Cl + Si (CH3)2SiCl2 The reaction of (CH3)2SiCl2, dichlorodimethylsilane, with water produces an interesting compound, dimethylsilanol, (CH3)2Si (OH)2. Dimethylsilanol under- goes a polymerization reaction in which H₂O molecules are eliminated from among large numbers of silanol molecules. The result of this polymerization is a material consisting of molecules with long silicon-oxygen chains: silicones. Hexasilane -H₂O CH3 CH3 CH3 →HO-Si-O-Si-o-Si-OH CH3 CH3 CH3 A silicone n
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
The reaction of CH33SiCl with water can be represented by the following equation CH33Si...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
China Petroleum and Chemical Corporation China Petroleum and Chemical Corporation (CPCC) is one of a growing number of Chinese companies that has cross-listed its stock on foreign stock exchanges. To...
-
Fluid flows in pipe networks can be analyzed in a manner similar to that used for electric resistance networks. Figure (a) shows a network with three pipes, which is analogous to the electrical...
-
Fluid flows in pipe networks can be analyzed in a manner similar to that used for electric resistance networks. Figure P6 shows a network with three pipes. The volume flow rates in the pipes are q 1...
-
What constant should replace the question mark in this system so that the solution set is {(1, 1, 1)}? 2x - 3y + -5x + 2y z = 0 - 2 = x + y + 2z = ? 4 -4
-
Why is the effective-interest amortization method theoretically superior to the straight-line method?
-
An 1800-N load Q is applied to the pulley C, which can roll on the cable ACB. The pulley is held in the position shown by a second cable CAD, which passes over the pulley A and supports a load P....
-
For the first 50 years of business, the Johnson Carpet Company produced carpets for residential use. The salesforce was structured geographically. In the past five years, a large percentage of carpet...
-
Income statements for Thompson Company for 2011 and 2012 follow. Required a. Perform a horizontal analysis, showing the percentage change in each income statement component between 2011 and 2012. b....
-
Andre's Kitchen is an all-equity firm with 500,000 shares outstanding at $25/share. The CFO of the company has just decided to take on $5 million in constant and perpetual debt to repurchase shares....
-
Use data from Appendix D (Table D-2) to estimate the minimum voltage required to electrolyze Al 2 O 3 in the Hall-Hroult process, reaction (21.25). Use f G[Al 2 O 3 (l)] = 1520 kJ mol 1 . Show that...
-
One way of distinguishing ionic behavior from covalent behavior is by comparing melting points. Ionic compounds tend to have higher melting points than covalent compounds. Which of the two halides of...
-
A popular alternative design for a positive- edge- triggered D lip- lop is shown in Figure 4-47. Manually or automatically simulate the circuit to determine whether its functional behavior is...
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,200 cases of Oktoberfest-style beer from a German supplier for 264,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Finding Confidence Intervals. In Exercises 9-16, assume that each sample is a simple random sample obtained from a population with a normal distribution. Body Temperature Data Set 5 "Body...
-
19 Part 2 of 2 1.25 points Skipped Required information Problem 6-4A & 6-5A (Algo) [The following information applies to the questions displayed below.] Gerald Utsey earned $48,400 in 2021 for a...
-
Describe equilibrium constants with words and equations. is the ratio of the concentrations of products to the concentration of reactants present in a reaction mixture when chemical equilibrium is...
-
Pronghorn Inc. acquired 20% of the outstanding common shares of Gregson Inc. on December 31, 2019. The purchase price was $1,133,000 for 51,500 shares, and is equal to 20% of Gregson's carrying...
-
Write a computer program (or develop an algorithm) to list the integer solutions for (a) x1 + x2 + x3 = 10, 0 xi, 1 i 3 (b) x1 + x2 + x3 + x4 = 4, - 2 xi, 1 i 4
-
Inexhaustible collections of ONPOs are not required to be capitalized or depreciated, if certain criteria are met. Why is this so, and what accounting and reporting recognition, if any, is required...
-
A mutual fund manager has a $20 million portfolio with a beta of 1.5. The risk-free rate is 4.5%, and the market risk premium is 5.5%. The manager expects to receive an additional $5 million, which...
-
Suppose you won the lottery and had two options: (1) receiving $0.5 million or (2) taking a gamble in which at the flip of a coin you receive $1 million if a head comes up but receive zero if a tail...
-
Stock X has a 10% expected return, a beta coefficient of 0.9, and a 35% standard deviation of expected returns. Stock Y has a 12.5% expected return, a beta coefficient of 1.2, and a 25% standard...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


