Question: Use data from Appendix D (Table D-2) to estimate the minimum voltage required to electrolyze Al 2 O 3 in the Hall-Hroult process, reaction (21.25).
Use data from Appendix D (Table D-2) to estimate the minimum voltage required to electrolyze Al2O3 in the Hall-Héroult process, reaction (21.25). Use ΔfG°[Al2O3(l)] = –1520 kJ mol–1. Show that the oxidation of the graphite anode to CO2(g) permits the electrolysis to occur at a lower voltage than if the electrolysis reaction were Al2O3(l) → 2 Al(l) + 3/2O2(g).
Table D-2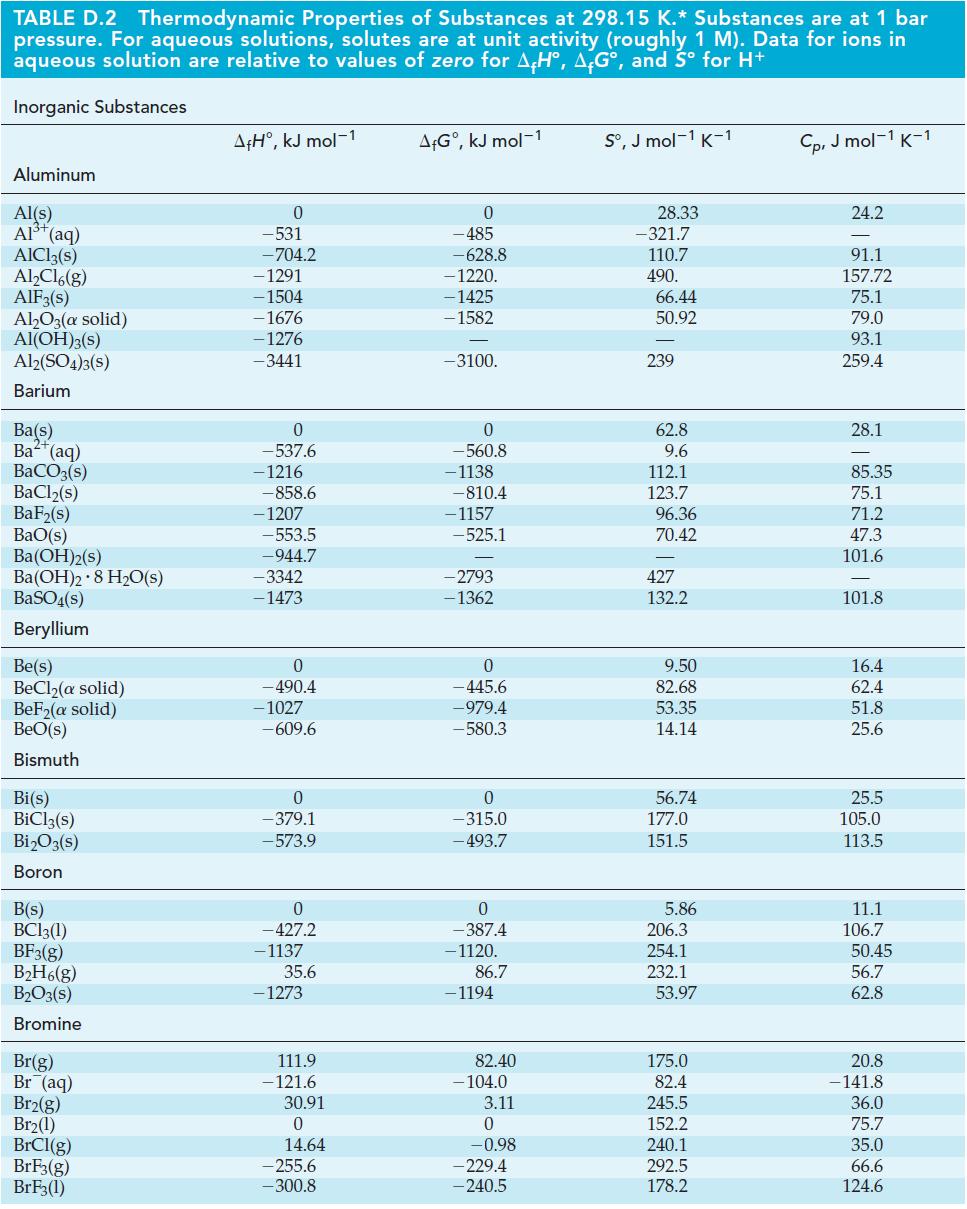
Reaction (21.25)
![]()
TABLE D.2 Thermodynamic Properties of Substances at 298.15 K.* Substances are at 1 bar pressure. For aqueous solutions, solutes are at unit activity (roughly 1 M). Data for ions in aqueous solution are relative to values of zero for AH, AG, and S for H+ Inorganic Substances Aluminum Al(s) Al+ (aq) AlCl3(s) AlCl6(g) AlF3(s) AlO3(a solid) Al(OH)3(s) Al2(SO4)3(S) Barium Ba(s) Ba+ (aq) BaCO3(s) BaCl(s) BaF(s) BaO(s) BA(OH)2(s) Ba(OH)2 8 HO(s) BaSO4(s) Beryllium Be(s) BeCl(a solid) BeF(a solid) BeO(s) Bismuth Bi(s) BiCl3(s) BiO3(s) Boron B(s) BC13(1) BF3(g) BH6(g) BO3(s) Bromine Br(g) Br (aq) Br2(g) Br(1) BrCl(g) BrF3(g) BrF3(1) A+H, kJ mol-1 0 -531 -704.2 -1291 -1504 -1676 -1276 -3441 0 -537.6 - 1216 -858.6 -1207 -553.5 -944.7 -3342 -1473 0 - 490.4 -1027 -609.6 0 -379.1 -573.9 0 -427.2 - 1137 35.6 - 1273 111.9 -121.6 30.91 0 14.64 -255.6 -300.8 A+G, kJ mol-1 0 -485 -628.8 -1220. -1425 - 1582 -3100. 0 -560.8 -1138 -810.4 -1157 -525.1 -2793 -1362 0 -445.6 -979.4 -580.3 0 -315.0 - 493.7 0 -387.4 -1120. 86.7 - 1194 82.40 -104.0 3.11 0 -0.98 -229.4 -240.5 S, J mol-1 K-1 28.33 -321.7 110.7 490. 66.44 50.92 239 62.8 9.6 112.1 123.7 96.36 70.42 427 132.2 9.50 82.68 53.35 14.14 56.74 177.0 151.5 5.86 206.3 254.1 232.1 53.97 175.0 82.4 245.5 152.2 240.1 292.5 178.2 Cp, J mol-1 K-1 24.2 91.1 157.72 75.1 79.0 93.1 259.4 28.1 85.35 75.1 71.2 47.3 101.6 101.8 16.4 62.4 51.8 25.6 25.5 105.0 113.5 11.1 106.7 50.45 56.7 62.8 20.8 - 141.8 36.0 75.7 35.0 66.6 124.6
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


