In each of the following examples, sketch a voltaic cell that uses the given reaction. Label the
Question:
In each of the following examples, sketch a voltaic cell that uses the given reaction. Label the anode and cathode; indicate the direction of electron flow; write a balanced equation for the cell reaction; and calculate E°cell.
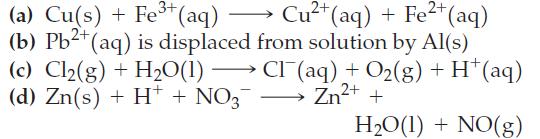
Transcribed Image Text:
3+ (a) Cu(s) + Fe³+ (aq) → Cu²+ (aq) + Fe²+ (aq) (b) Pb²+ (aq) is displaced from solution by Al(s) (c) Cl₂(g) + H₂O(1) →→→→CI (aq) + O₂(g) + H+ (aq) (d) Zn(s) + H+ + NO3¯¯ → Zn²+ + H₂O(l) + NO(g)
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) Pd(s) + 4 Cl- (aq) + Cd2+ (aq) has a measured standard cell potential of + 1.03 V. (a) Write the two half-cell reactions. (b) By using data...
-
A voltaic cell that uses the reaction Tl3+(aq) + 2 Cr2+(aq) Tl+(aq) + 2 Cr3+(aq) has a measured standard cell potential of + 1.19 V. (a) Write the two half-cell reactions. (b) By using data from...
-
Read the statement below and answer all five questions that follow: The procurement professional task is to reduce the cost of a product, material or service through supplier relationship development...
-
Lacoste t-shirts come with an average price of $ 120 a piece, at their factory outlet with a std. deviation of $ 17. But at the Seasonal Sale (Discount) outlets of these t- shirts, it was also...
-
You are the sales manager of a small company with sales in the United States. About 30 percent of your business is mail order, and the remainder is from your two retail stores. You recently created...
-
A taxpayer can invest $ 10,000 in a taxable 10-year bond that yields an annual pre-tax return of 6 percent or buy land (a capital asset) for $ 10,000 that is expected to increase at an annual pre-tax...
-
Speed Limit A county is considering raising the speed limit on a road because they claim that the mean speed of vehicles is greater than 45 miles per hour. A random sample of 25 vehicles has a mean...
-
A small immersion heater is rated at 350 W. Estimate how long it will take to heat a cup of soup (assume this is 250mL of water) from 20oC to 60oC.
-
A stock has an expected return of 13.2 percent, its beta is .70, and the risk-free rate is 3.3 percent. What must the expected return on the market be? (Do not round intermediate calculations. Enter...
-
Write the equilibrium constant expression for each of the following reactions, and determine the value of K at 25 C. Use data from Table 19.1. Table 19.1 2 V (a) 2 V+ (aq) + Ni(s) > (b) MnO (s) + 4...
-
Predict whether, to any significant extent, (a) Fe(s) will displace Zn 2+ (aq); (b) MnO 4 - (aq) will oxidize Cl - (aq) to Cl 2 (g) in acidic solution; (c) Ag(s) will react with 1 M HCl(aq); (d) O 2...
-
Use standard Gibbs energies of formation to calculate the standard reaction Gibbs energies at 298 K of the reactions in Exercise 3.8b.
-
Why do you think it is important to consider only relevant costs when conducting a differential analysis for a major purchase? Why not consider all possible costs in your decision? provide specific...
-
How do power dynamics and influence tactics shape decision-making processes and organizational politics within hierarchical structures ?
-
How do I answer these given the information below? Loan Amount? Loan to Value? Loan to Cost? Payment amount? Loan Balance at Maturity? Given Information: Property Cost: $1,000,000 Bank Policy on LTV:...
-
In your initial post, first do the following: Use scholarly references to define Project Management (PM), Systems Development Life Cycle (SDLC), and Application Life Cycle (AL). Then, in the same...
-
How do concepts of diversity and inclusion vary across different cultural and geographical contexts, and what strategies can multinational organizations employ to navigate these variations...
-
Facebook Inc. included the following disclosure note in an annual report: Share-Based Compensation (in part) . . . compensation expense related to these grants is based on the grant date fair value...
-
F.(3e* -2x 3 sin(2x)) is equal to 2 3 Cos 8. IT 3, t (4+@ 2 3, 1+o 1 4 Cos 4 4 1 3. 1 +4cos V7 (1+o 4 1 4 Cos 4 1+0 4-
-
Give the formulas under the direct method for computing (a) Cash receipts from customers and (b) Cash payments to suppliers.
-
Garcia Inc. reported sales of $2 million for 2010.Accounts receivable decreased $200,000 and accounts payable increased $300,000. Compute cash receipts from customers, assuming that the receivable...
-
In its 2007 statement of cash flows, what amount did PepsiCo report for net cash (a) Provided by operating activities, (b) Used for investing activities, and (c) Used for financing activities?
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


