In ionic compounds with certain metals, hydrogen exists as the hydride ion, H - . Determine the
Question:
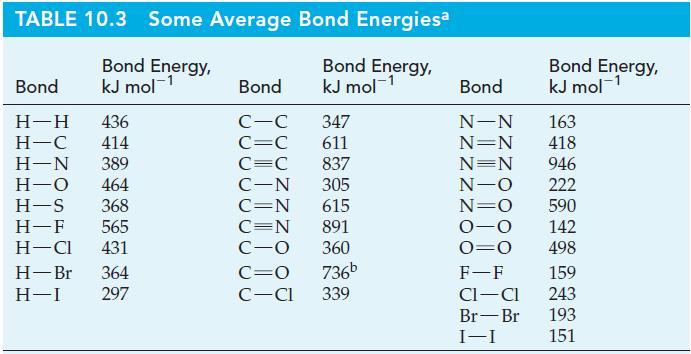
In ionic compounds with certain metals, hydrogen exists as the hydride ion, H-. Determine the electron affinity of hydrogen; that is, ΔrH for the process H(g) + e- : H-(g). The bond energy of H2(g) from Table 10.3; -812 kJ mol-1 for the lattice energy of NaH(s); and -57 kJ mol-1 NaH for the enthalpy of formation of NaH(s).
Table 10.3
Transcribed Image Text:
TABLE 10.3 Some Average Bond Energiesa Bond Energy, kJ mol-¹ Bond Energy, kJ mol-¹ Bond H-H 436 H-C 414 H-N 389 H-O 464 H-S 368 H-F 565 H-Cl 431 H-Br 364 H-I 297 Bond C-C 347 C=C 611 C=C 837 C-N 305 C=N 615 C=N 891 C-O 360 C=O 736b C-Cl 339 Bond N-N N=N N=N N-O N=O 0-0 0=0 F-F CI-CI Br-Br I-I Bond Energy, kJ mol-¹ 163 418 946 222 590 142 498 159 243 193 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To determine the electron affinity of hydrogen we can use the following ...View the full answer

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The lattice energy of an ionic solid such as NaCl is the enthalpy change H° for the process in which the solid changes to ions. For example, NaCl(s) Na+(g) + Cl(g) H = 786 kJ/mol Assume that the...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Hydrogen is an unusual element because it behaves in some ways like the alkali metal elements and in other ways like nonmetals. Its properties can be explained in part by its electron configuration...
-
Who was the petitioner? Who was the respondent? b. In what year was the case heard? c. What tax years did the case address? d. Who was the judge in the case? e. What was the basic issue in the case?...
-
On March 10, Tolliver Tolles, also known as Thomas Towle, delivered to Alonzo Craig and Abigail Craig the following instrument, written by him in pencil: For value received, I, Thomas Towle, promise...
-
Dinh and Bill are involved in an automobile accident. Sue is a passenger in Bills car. Dinhs attorney wants to ask Sue, as a witness, some questions concerning the accident. Sues answers to the...
-
Evaluate instances of mental model failure/trauma that led to changes in your mental model.
-
Jim Smith's dealership sells Fords, Hondas, and Toyotas. The dealership keeps information about each car manufacturer with whom it deals so that employees can get in touch with manufacturers easily....
-
Please help explaining both question a and b. FIFO and LIFO Costs Under Perpetual Inventory System The following units of an item were available for sale during the year: Beginning inventory 41 units...
-
Deduce the structure of a compound with molecular formula C 5 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. Data from the mass spectrum are also provided. 100 Mass Spec. Data...
-
Determine the lattice energy of KF(s) from the following data: f H[KF(s)] = -567.3 kJ mol -1 ; enthalpy of sublimation of K(s), 89.24 kJ mol -1 ; enthalpy of dissociation of F 2 (g), 159 kJ mol -1 F...
-
Without doing calculations, indicate how you would expect the lattice energies of LiCl(s), KCl(s), RbCl(s), and CsCl(s) to compare with the value of -787 kJ mol -1 determined for NaCl(s).
-
On March 31, 2015, Easy Rental Agency Inc.'s trial balance included the following unadjusted account balances. The company's year end is December 31 and it adjusts its accounts quarterly. An analysis...
-
Explain the process of compression resin transfer molding(CRTM)?in composite manufacturing. What are the benefits of using CRTM for producing composite structures?
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Discuss the principles of geotechnical engineering in slope stability analysis. How can engineers assess slope stability, mitigate landslide risks, and design effective stabilization measures to...
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Briefly explain what spatial autocorrelation means and what method can be used to measure it
-
Draw the structure of a compound with each of the following molecular formulas that will show only one peak in its 1H NMR spectrum. a. C6H12 b. C3H6Cl2 c. C12H18 d. C6H6 e. C5H12 f. C2H6S
-
CLASS PERIO Solving Linear Equations: Variable on Both Sides Solve each equation. 1) 6r+ 7 = 13 + 7r 3) -7x-3x+2=-8x-8 5)-14 +66+7-26=1+5b 7) n-3n = 14-4n 2) 13-4x=1-x 4)-8-x= x - 4x 6)n+2=-14-n 8)...
-
APR and EAR should lending laws be changed to require lenders to report EARs instead of APRs? Why or why not?
-
Time Value On subsidized Stafford loans a common source of financial aid for college students, interest does not begin to accrue until repayment begins. Who receives a bigger subsidy, a freshman or a...
-
Time Value Eligibility for a subsidized Stafford loan is based on current financial need. However, both subsidized and unsubsidized Stafford loans are repaid out of future income. Given this, do you...
-
A BOND IS SELLING AT 777 DOLLAR AND COUPON RATE IS 7% WHAT IS THE CURRENT YIELD ROUND THE NUMBER TO 2 DECIMALS (0.00)
-
ABC Corporation gathered the following information relating to its inventories: (A) Inventories per physical count P3,000,000; (B) Inventories consigned to ABC included in the count P100,000; (C)...
-
Documenti Word Layout References 1.ailing: Rei View Help Tell me you want to do 53. You are to read the attached notes and develop two items that will be added to the answer sheet under question 74...

Study smarter with the SolutionInn App


