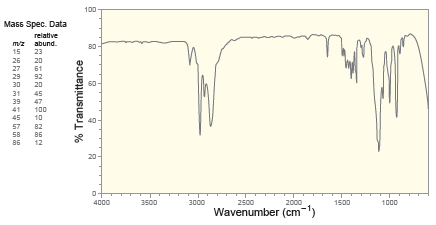
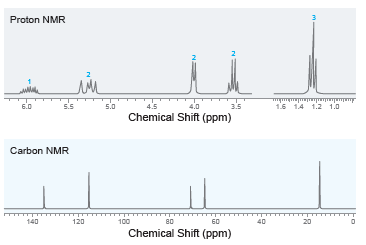
Deduce the structure of a compound with molecular formula C 5 H 10 O that exhibits the
Question:
Transcribed Image Text:
100 Mass Spec. Data relative mz abund. 15 23 26 27 29 20 61 92 30 20 31 39 47 41 100 45 10 57 82 58 86 86 12 20 4000 3500 3000 2500 2000 1500 1000 Wavenumber (cm-") % Transmittance Proton NMR 1.5 14 1.2 1.0 6.0 5.5 5.0 4.5 4.0 3.5 Chemical Shift (ppm) Carbon NMR 140 120 100 80 60 20 Chemical Shift (ppm)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)

Answered By

Cristine kanyaa
I possess exceptional research and essay writing skills. I have successfully completed over 5000 projects and the responses are positively overwhelming . I have experience in handling Coursework, Session Long Papers, Manuscripts, Term papers, & Presentations among others. I have access to both physical and online library. this makes me a suitable candidate to tutor clients as I have adequate materials to carry out intensive research.
4.90+
1538+ Reviews
3254+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Deduce the structure of a compound with molecular formula C 9 H 10 O 2 that produces the following 1 H NMR spectrum and 13 C NMR spectrum: Proton NMR 10 Chemical Shift (ppm) Carbon NMR - 128.4 128.8-...
-
The IR spectrum of a compound with molecular formula C5H8O was obtained in CCl4 and is shown in Figure 13.42. Identify the compound. Wavelenga qum) 15 16 14 3600 340) 3800 3300 3000 280K 2600 2400...
-
The 1 H NMR spectrum of a compound with molecular formula C 7 H 15 C l exhibits two signals with relative integration 2 : 3. Propose a structure for this compound.
-
You have been given the following algorithm. What does it return? Input: Array A [ 1 . . . [ 1 . . . n ] ] Output: ? ? ? ? out 1 1 for i 2 2 to n do if A [ [ i ] < ] < A [ [ out ] ] then out i return...
-
What is cyber squatting?
-
In Exercises find the indefinite integral. S sin 2x cos 2x dx
-
6. Many companies hold significant amounts of excess cash, or cash above the amount required for day-to-day operations. What would happen to the ROIC for HealthCo if you included excess cash in its...
-
PRJ Candy Company makes and sells two kinds of candy bars: chocolate almond and coconut. During the past several years, the company has kept accurate records of costs and resource requirements and...
-
Digital Electronics Inc. manufactures computer monitors, which includes a power supply. Digital's current costs of manufacturing the 10,000 power supply units needed per month are: Direct materials...
-
Gallium has an orthorhombic structure with a0 = 0.45258 nm, b0 = 0.45186 nm, and c0 = 0.76570 nm. The atomic radius is 0.1218 nm. The density is 5.904 g/cm3, and the atomic weight is 69.72 g/mol....
-
Deduce the structure of a compound with molecular formula C 8 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. 100 80 60 40 20 2500 2000 Wavenumber (cm-1) 1000 4000 3500 3000...
-
For each of the following compounds, determine whether the two protons shown in red are homotopic, enantiotopic, or diastereotopic: (a) (b) (c) (d) (e) Discuss. OMe . CI H,
-
A regional manager at Sears compares customer satisfaction ratings (1, 2, 3, or 4 stars) at the companys Media, Pennsylvania, store (M); Exton, Pennsylvania, store (E); and Darby, Pennsylvania, store...
-
Classic Auto Parts sells new and used auto parts. Although a majority of its sales are cash sales, it makes a significant amount of credit sales. During 2012, its first year of operations, Classic...
-
The following information is available for Market Inc. and Supply Inc. at December 31, 2012: Required a. What is the accounts receivable turnover for each of the companies for 2012 ? b. What is the...
-
Buck Novak, the chief executive officer of Novak Corporation, has assembled his top advisers to evaluate an investment opportunity. The advisers expect the company to pay \($400,000\) cash at the...
-
Verify the log-likelihood in equation (16.4) for the Tobit model. In L = = In { 1-0 (x-di)} 1:y=di 122. + (y; - x) 02 (16.4) i:y;>di
-
Milo Company is considering the purchase of new equipment for its factory. It will cost \($250,000\) and have a \($50,000\) salvage value in five years. 1 he annual net income from the equipment is...
-
Make a scatterplot of the data. Then find an exponential, logarithmic, or logistic function f that best models the data. r 0 0.3 1 1.3 2 4.0 3 7.5 4 9.3 5 9.8
-
Cobb Manufacturing Company uses a process cost system and average costing. The following production data is for the month of June 2011. Production Costs Work in process, beginning of the month:...
-
Draw and name the 17 hexene isomers, C6H2, including E, Z isomers.
-
Trans-2-Buterw is more stable than cic-2-hutene by only 4kJ/mol, but trans-2, 2, 5; 5-tetramethyl-3-hexene is more stable than its cis isomer by 3kJ/mol. Explain.
-
Cyclodecene can exist in both cis and Trans forms, but cyclohexene cannot. Explain. (Making molecular models is helpful.)
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


