Deduce the structure of a compound with molecular formula C 9 H 10 O 2 that produces
Question:
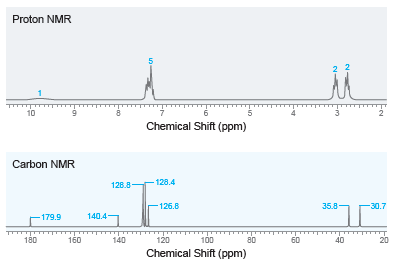
Transcribed Image Text:
Proton NMR 10 Chemical Shift (ppm) Carbon NMR - 128.4 128.8- 35.8 -126.8 -30.7 140.4 -179.9 180 160 100 140 120 80 60 40 20 Chemical Shift (ppm)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)

Answered By

YOGENDRA NAILWAL
As I'm a Ph.D. student, so I'm more focussed on my chemistry laboratory. I have qualified two national level exams viz, GATE, and NET JRF (Rank 68). So I'm highly qualified in chemistry subject. Also, I have two years of teaching experience in this subject, which includes college teacher as well as a personal tutor. I can assure you if you hire me on this particular subject, you are never going to regret it.
Best Regards.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the structure of a compound with molecular formula C 5 H 12 that exhibits only one kind of proton. That is, all 12 protons are chemically equivalent.
-
The IR spectrum of a compound with molecular formula C5H8O was obtained in CCl4 and is shown in Figure 13.42. Identify the compound. Wavelenga qum) 15 16 14 3600 340) 3800 3300 3000 280K 2600 2400...
-
Deduce the structure of a compound having the mass spectrum and 1H NMR spectrum presented in Figure 13.43.
-
Select the reasons why most professional telescopes are reflectors rather than refractors. A mirror can collect light more efficiently than a lens. Reflecting telescopes have shorter focal lengths...
-
1. According to the case, how many Yahoo!'s user accounts were stolen in 2013? 2. What types of information were stolen? 3. When did Yahoo! find out about the 1 billion user accounts breach? 4....
-
This question suggest that you think about various ethical dilemmas and how you would face them, reflect on situations you've faced, and learn from others. Increasing mindfulness about these...
-
What are the points of difference, or unique attributes, for X-1 products?
-
Midland Oil has $1,000 par value bonds outstanding at 8 percent interest. The bonds will mature in 25 years. Compute the current price of the bonds if the present yield to maturity is: a. 7% b. 10%...
-
On April 1, 2020, Paul sold a house to Amy. The property tax on the house, which is based on a calendar year, was due September 1, 2020. Amy paid the full amount of property tax of $2,500. Required:...
-
The marketing manager of a large truck manufacturer was surprised to learn that the price lists generated by his department had little relation to the prices that were actually charged to customers....
-
Why does species richness vary from one community to another?
-
Look at Figure 1.3. Suppose that the cost of cheese falls, so that the marginal cost of producing pizza decreases. Will the MC curve shift up or down? Will the optimal amount of pizza increase or...
-
p. 510 Organizational restructuring efforts have a weak negative effect on job performance. They have a more significant negative effect on organizational commitment because employees tend to feel...
-
Aircraft \(B\) has a constant speed of \(150 \mathrm{~m} / \mathrm{s}\) as it passes the bottom of a circular loop of 400-m radius. Aircraft \(A\) flying horizontally in the plane of the loop passes...
-
A small inspection car with a mass of \(200 \mathrm{~kg}\) runs along the fixed overhead cable and is controlled by the attached cable at \(A\). Determine the acceleration of the car when the control...
-
An aircraft \(P\) takes off at \(A\) with a velocity \(v_{0}\) of \(250 \mathrm{~km} / \mathrm{h}\) and climbs in the vertical \(y^{\prime}-z^{\prime}\) plane at the constant \(15^{\circ}\) angle...
-
If each resistor in Figure P31.75 has resistance \(R=5.0 \Omega\), what is the equivalent resistance of the combination? Data from Figure P31.75 wwwwww wwwww www www wwwww
-
Identify the proper point to recognize expense for each of the following transactions. a. Kat Inc. purchases on credit six custom sofas for \(\$ 800\) each in June. Two of the sofas are sold for \(\$...
-
The population of California in millions x years after 2010 is modeled by P(x) = 37.3e 0.01x . (a) Evaluate P(2). Interpret this result. (b) Find the y-intercept on the graph of y = P(x). Interpret...
-
Hardin Services Co. experienced the following events in 2016: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
Predict the products of the following reactions: (b) CH CHCCHH2CH (a) HCI H (d) CH CH2 (c) HBr CH-CH3CH2 H20 H2SO, (Addition of H20 occurs.)
-
What alkenes would you start with to prepare the following alkylhalides? CH-CH (a) (b) Br Br CI (c) (d) CH3CH2CHCH2CH2CH3
-
Show the structures of the carbocation intermediates you would expect in the following reactions: (b) CH3 (a) CH3CH2CCHCH3 HI CH H Er
-
As the representative of the local accounting club, you have been asked by the dean to help her understand the costs of the different degrees offered at the school. You decide to use an...
-
Gallatin Carpet Cleaning has always charged a flat fee per hundred square feet of carpet cleaned. The current fee is $ 1 2 . 6 0 per hundred square feet. However, there is some question about whether...
-
Lindell, Inc. has 8% , $100 par value preferred stock outstanding. To earn 12% on an investment in this stock, you need to purchase the shares at a per share price of $66.67 77.30 Ivonne has bought...

Study smarter with the SolutionInn App


